Oxygen limitation within a bacterial aggregate
- PMID: 24736225
- PMCID: PMC3994514
- DOI: 10.1128/mBio.00992-14
Oxygen limitation within a bacterial aggregate
Abstract
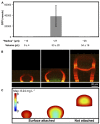
ABSTRACT Cells within biofilms exhibit physiological heterogeneity, in part because of chemical gradients existing within these spatially structured communities. Previous work has examined how chemical gradients develop in large biofilms containing >10(8) cells. However, many bacterial communities in nature are composed of small, densely packed aggregates of cells (≤ 10(5) bacteria). Using a gelatin-based three-dimensional (3D) printing strategy, we confined the bacterium Pseudomonas aeruginosa within picoliter-sized 3D "microtraps" that are permeable to nutrients, waste products, and other bioactive small molecules. We show that as a single bacterium grows into a maximally dense (10(12) cells ml(-1)) clonal population, a localized depletion of oxygen develops when it reaches a critical aggregate size of ~55 pl. Collectively, these data demonstrate that chemical and phenotypic heterogeneity exists on the micrometer scale within small aggregate populations. IMPORTANCE Before developing into large, complex communities, microbes initially cluster into aggregates, and it is unclear if chemical heterogeneity exists in these ubiquitous micrometer-scale aggregates. We chose to examine oxygen availability within an aggregate since oxygen concentration impacts a number of important bacterial processes, including metabolism, social behaviors, virulence, and antibiotic resistance. By determining that oxygen availability can vary within aggregates containing ≤ 10(5) bacteria, we establish that physiological heterogeneity exists within P. aeruginosa aggregates, suggesting that such heterogeneity frequently exists in many naturally occurring small populations.
Figures



References
-
- Walters MC, III, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47:317–323. 10.1128/AAC.47.1.317-323.2003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

