KCTD1 suppresses canonical Wnt signaling pathway by enhancing β-catenin degradation
- PMID: 24736394
- PMCID: PMC3988066
- DOI: 10.1371/journal.pone.0094343
KCTD1 suppresses canonical Wnt signaling pathway by enhancing β-catenin degradation
Retraction in
-
Retraction: KCTD1 Suppresses Canonical Wnt Signaling Pathway by Enhancing β-catenin Degradation.PLoS One. 2022 May 12;17(5):e0268604. doi: 10.1371/journal.pone.0268604. eCollection 2022. PLoS One. 2022. PMID: 35550646 Free PMC article. No abstract available.
Abstract
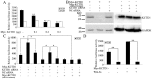
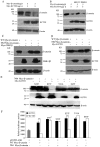
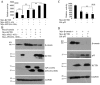
The canonical Wnt signaling pathway controls normal embryonic development, cellular proliferation and growth, and its aberrant activity results in human carcinogenesis. The core component in regulation of this pathway is β-catenin, but molecular regulation mechanisms of β-catenin stability are not completely known. Here, our recent studies have shown that KCTD1 strongly inhibits TCF/LEF reporter activity. Moreover, KCTD1 interacted with β-catenin both in vivo by co-immunoprecipitation as well as in vitro through GST pull-down assays. We further mapped the interaction regions to the 1-9 armadillo repeats of β-catenin and the BTB domain of KCTD1, especially Position Ala-30 and His-33. Immunofluorescence analysis indicated that KCTD1 promotes the cytoplasmic accumulation of β-catenin. Furthermore, protein stability assays revealed that KCTD1 enhances the ubiquitination/degradation of β-catenin in a concentration-dependent manner in HeLa cells. And the degradation of β-catenin mediated by KCTD1 was alleviated by the proteasome inhibitor, MG132. In addition, KCTD1-mediated β-catenin degradation was dependent on casein kinase 1 (CK1)- and glycogen synthase kinase-3β (GSK-3β)-mediated phosphorylation and enhanced by the E3 ubiquitin ligase β-transducin repeat-containing protein (β-TrCP). Moreover, KCTD1 suppressed the expression of endogenous Wnt downstream genes and transcription factor AP-2α. Finally, we found that Wnt pathway member APC and tumor suppressor p53 influence KCTD1-mediated downregulation of β-catenin. These results suggest that KCTD1 functions as a novel inhibitor of Wnt signaling pathway.
Conflict of interest statement
Figures






References
-
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810. - PubMed
-
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A (2004) WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 5: 691–701. - PubMed
-
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, et al. (1998) Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

