Cobalt protoporphyrin pretreatment protects human embryonic stem cell-derived cardiomyocytes from hypoxia/reoxygenation injury in vitro and increases graft size and vascularization in vivo
- PMID: 24736402
- PMCID: PMC4039456
- DOI: 10.5966/sctm.2013-0189
Cobalt protoporphyrin pretreatment protects human embryonic stem cell-derived cardiomyocytes from hypoxia/reoxygenation injury in vitro and increases graft size and vascularization in vivo
Abstract
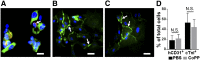
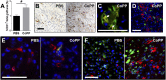
Human embryonic stem cell-derived cardiomyocytes (hESC-CMs) can regenerate infarcted myocardium. However, when implanted into acutely infarcted hearts, few cells survive the first week postimplant. To improve early graft survival, hESC-CMs were pretreated with cobalt protoporphyrin (CoPP), a transcriptional activator of cytoprotective heme oxygenase-1 (HO-1). When hESC-CMs were challenged with an in vitro hypoxia/reoxygenation injury, mimicking cell transplantation into an ischemic site, survival was significantly greater among cells pretreated with CoPP versus phosphate-buffered saline (PBS)-pretreated controls. Compared with PBS-pretreated cells, CoPP-pretreated hESC-CM preparations exhibited higher levels of HO-1 expression, Akt phosphorylation, and vascular endothelial growth factor production, with reduced apoptosis, and a 30% decrease in intracellular reactive oxygen species. For in vivo translation, 1 × 10(7) hESC-CMs were pretreated ex vivo with CoPP or PBS and then injected intramyocardially into rat hearts immediately following acute infarction (permanent coronary ligation). At 1 week, hESC-CM content, assessed by quantitative polymerase chain reaction for human Alu sequences, was 17-fold higher in hearts receiving CoPP- than PBS-pretreated cells. On histomorphometry, cardiomyocyte graft size was 2.6-fold larger in hearts receiving CoPP- than PBS-pretreated cells, occupying up to 12% of the ventricular area. Vascular density of host-perfused human-derived capillaries was significantly greater in grafts composed of CoPP- than PBS-pretreated cells. Taken together, these experiments demonstrate that ex vivo pretreatment of hESC-CMs with a single dose of CoPP before intramyocardial implantation more than doubled resulting graft size and improved early graft vascularization in acutely infarcted hearts. These findings open the door for delivery of these, or other, stem cells during acute interventional therapy following myocardial infarction or ischemia.
Keywords: Acute myocardial infarction; Cell therapy; Heme oxygenase-1; Human embryonic stem cell; Infarct repair; Preconditioning.
©AlphaMed Press.
Figures
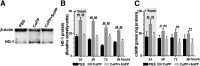


References
-
- Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. - PubMed
-
- Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. - PubMed
-
- Li RK, Mickle DA, Weisel RD, et al. Optimal time for cardiomyocyte transplantation to maximize myocardial function after left ventricular injury. Ann Thorac Surg. 2001;72:1957–1963. - PubMed
-
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

