Absence of metalloprotease GP63 alters the protein content of Leishmania exosomes
- PMID: 24736445
- PMCID: PMC3988155
- DOI: 10.1371/journal.pone.0095007
Absence of metalloprotease GP63 alters the protein content of Leishmania exosomes
Abstract
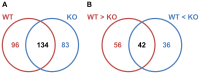
Protozoan parasites of Leishmania genus are able to successfully infect their host macrophage due to multiple virulence strategies that result in its deactivation. Recent studies suggest Leishmania GP63 to be a critical virulence factor in modulation of many macrophage molecules, including protein tyrosine phosphatases (PTPs) and transcription factors (TFs). Additionally, we and others recently reported that Leishmania-released exosomes can participate in pathogenesis. Exosomes are 40-100 nm vesicles that are freed by many eukaryotic cells. To better understand the GP63-dependent immune modulation of the macrophage by Leishmania parasites and their exosomes, we compared the immunomodulatory properties of Leishmania major (WT) and L. major gp63-/- (KO) as well as their exosomes in vitro and in vivo. Importantly, we observed that Leishmania exosomes can modulate macrophage PTPs and TFs in a GP63-dependent manner. In addition, our qRT-PCR analyses showed that WT parasites were able to downregulate multiple genes involved in the immune response, especially cytokines and pattern recognition receptors. KO parasites showed a strongly reduced modulatory capacity compared to WT parasites. Furthermore, comparison of WT versus KO exosomes also showed divergences in alteration of gene expression, especially of chemokine receptors. In parallel, studying the in vivo inflammatory recruitment using a murine air pouch model, we found that exosomes have stronger proinflammatory properties than parasites and preferentially induce the recruitment of neutrophils. Finally, comparative proteomics of WT and KO exosomes surprisingly revealed major differences in their protein content, suggesting a role for GP63 in Leishmania exosomal protein sorting. Collectively our data clearly establish the crucial role of GP63 in dampening the innate inflammatory response during early Leishmania infection, and also provides new insights in regard to the role and biology of exosomes in Leishmania host-parasite interactions.
Conflict of interest statement
Figures




References
-
- Kaye P, Scott P (2011) Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol 9: 604–615. - PubMed
-
- Yao C, Donelson JE, Wilson ME (2003) The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol Biochem Parasitol 132: 1–16. - PubMed
-
- Olivier M, Atayde VD, Isnard A, Hassani K, Shio MT (2012) Leishmania virulence factors: Focus on the metalloprotease GP63. Microbes and Infection. - PubMed
-
- Forget G, Gregory DJ, Olivier M (2005) Proteasome-mediated degradation of STAT1alpha following infection of macrophages with Leishmania donovani. J Biol Chem 280: 30542–30549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

