Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal
- PMID: 24736500
- PMCID: PMC3988488
- DOI: 10.1038/srep04706
Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal
Abstract
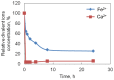
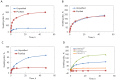
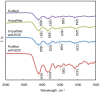
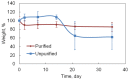
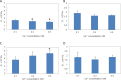
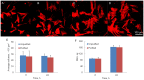
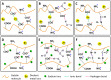
The usage of gelatin hydrogel is limited due to its instability and poor mechanical properties, especially under physiological conditions. Divalent metal ions present in gelatin such as Ca(2+) and Fe(2+) play important roles in the gelatin molecule interactions. The objective of this study was to determine the impact of divalent ion removal on the stability and mechanical properties of gelatin gels with and without chemical crosslinking. The gelatin solution was purified by Chelex resin to replace divalent metal ions with sodium ions. The gel was then chemically crosslinked by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC). Results showed that the removal of divalent metal ions significantly impacted the formation of the gelatin network. The purified gelatin hydrogels had less interactions between gelatin molecules and form larger-pore network which enabled EDC to penetrate and crosslink the gel more efficiently. The crosslinked purified gels showed small swelling ratio, higher crosslinking density and dramatically increased storage and loss moduli. The removal of divalent ions is a simple yet effective method that can significantly improve the stability and strength of gelatin hydrogels. The in vitro cell culture demonstrated that the purified gelatin maintained its ability to support cell attachment and spreading.
Figures



References
-
- Silva S. S., Mano J. F. & Reis R. L. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit. Rev. Biotechnol. 30, 200–221 (2010). - PubMed
-
- Huang S. & Fu X. B. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release 142, 149–159 (2010). - PubMed
-
- Neffe A. T. et al. Gelatin functionalization with tyrosine derived moieties to increase the interaction with hydroxyapatite fillers. Acta Biomater. 7, 1693–1701 (2011). - PubMed
-
- Yuan S. J., Xiong G., Roguin A. & Choong C. Immobilization of Gelatin onto Poly(Glycidyl Methacrylate)-Grafted Polycaprolactone Substrates for Improved Cell-Material Interactions. Biointerphases 7, 30 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

