Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial
- PMID: 24736628
- PMCID: PMC3988010
- DOI: 10.1371/journal.pone.0092843
Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial
Abstract
Objective: The aim of this study was to assess the glucose-lowering and lipid-modifying effects, and safety profile of lobeglitazone, a novel peroxisome proliferator-activated receptor- γ agonist, compared to placebo as a monotherapy in patients with type 2 diabetes.
Research design and methods: In this 24-week, multicenter, randomized, double-blind, parallel-group, placebo controlled study, 173 patients were randomly assigned (a 2∶1 ratio) to lobeglitazone 0.5 mg (n=115) or matching placebo (n=58) orally once daily. The primary endpoint was the change in glycated hemoglobin (HbA1c) from baseline to the end of treatment. The secondary endpoints included various glycemic parameters, lipid parameters and safety profile (ClinicalTrials.gov number NCT01001611).
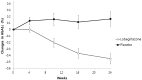
Results: At 24 weeks, a significant reduction in HbA1c was observed with lobeglitazone versus placebo (-0.44% vs 0.16%, mean difference -0.6%, p<0.0001). The goal of HbA1c <7% was achieved significantly more in the lobeglitazone group compared to the placebo group (44% vs 12%, p<0.0001). Markers of insulin resistance were also improved in the lobeglitazone group. In addition, lobeglitazone treatment significantly improved triglycerides, high density lipoprotein cholesterol, small dense low density lipoprotein cholesterol, free fatty acid, and apolipoprotein-B/CIII compared to placebo (p<0.01, respectively). More weight gain was observed in the lobeglitazone group than the placebo group (0.89 kg vs - 0.63 kg, mean difference 1.52 kg, p<0.0001). The safety profile was comparable between the two groups and lobeglitazone was well tolerated.
Conclusions: Lobeglitazone 0.5 mg showed a favorable balance in the efficacy and safety profile. The results support a potential role of lobeglitazone in treating type 2 diabetes.
Trial registration: Clinicaltrials.gov NCT01001611.
Conflict of interest statement
Figures
Similar articles
-
Safety and efficacy of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 52 weeks: An open-label extension study.Diabetes Res Clin Pract. 2015 Dec;110(3):e27-30. doi: 10.1016/j.diabres.2015.09.009. Epub 2015 Sep 21. Diabetes Res Clin Pract. 2015. PMID: 26458774 Clinical Trial.
-
Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension.Diabetes Obes Metab. 2015 Jun;17(6):599-602. doi: 10.1111/dom.12435. Epub 2015 Feb 8. Diabetes Obes Metab. 2015. PMID: 25580775 Free PMC article. Clinical Trial.
-
Randomized, Double-blind, Phase III Trial of Lobeglitazone Add-on to Metformin in Type 2 Diabetes (SENSITIZE INDIA).J Assoc Physicians India. 2024 Jan;72(1):32-42. doi: 10.59556/japi.71.0445. J Assoc Physicians India. 2024. PMID: 38736072 Clinical Trial.
-
Efficacy and safety of lobeglitazone, a new Thiazolidinedione, as compared to the standard of care in type 2 diabetes mellitus: A systematic review and meta-analysis.Diabetes Metab Syndr. 2023 Jan;17(1):102703. doi: 10.1016/j.dsx.2022.102703. Epub 2023 Jan 2. Diabetes Metab Syndr. 2023. PMID: 36634469
-
Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis.Diabetes Metab Syndr. 2023 Jan;17(1):102697. doi: 10.1016/j.dsx.2022.102697. Epub 2022 Dec 23. Diabetes Metab Syndr. 2023. PMID: 36580702 Review.
Cited by
-
Lobeglitazone, a novel thiazolidinedione, for secondary prevention in patients with ischemic stroke: a nationwide nested case-control study.Cardiovasc Diabetol. 2023 May 5;22(1):106. doi: 10.1186/s12933-023-01841-4. Cardiovasc Diabetol. 2023. PMID: 37147722 Free PMC article.
-
Chronic Inflammation in Non-Alcoholic Steatohepatitis: Molecular Mechanisms and Therapeutic Strategies.Front Endocrinol (Lausanne). 2020 Dec 14;11:597648. doi: 10.3389/fendo.2020.597648. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33384662 Free PMC article. Review.
-
Lobeglitazone and Its Therapeutic Benefits: A Review.Cureus. 2023 Dec 6;15(12):e50085. doi: 10.7759/cureus.50085. eCollection 2023 Dec. Cureus. 2023. PMID: 38186506 Free PMC article. Review.
-
Peroxisome Proliferator-Activated Receptors as a Therapeutic Target in Asthma.PPAR Res. 2020 Jan 14;2020:8906968. doi: 10.1155/2020/8906968. eCollection 2020. PPAR Res. 2020. PMID: 32395125 Free PMC article. Review.
-
Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks.Diabetes Metab J. 2017 Oct;41(5):377-385. doi: 10.4093/dmj.2017.41.5.377. Diabetes Metab J. 2017. PMID: 29086536 Free PMC article.
References
-
- Yki-Jarvinen H (2004) Thiazolidinediones. N Engl J Med 351: 1106–1118. - PubMed
-
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, et al. (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355: 2427–2443. - PubMed
-
- Deeg MA, Buse JB, Goldberg RB, Kendall DM, Zagar AJ, et al. (2007) Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30: 2458–2464. - PubMed
-
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, et al. (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366: 1279–1289. - PubMed
-
- Cariou B, Charbonnel B, Staels B (2012) Thiazolidinediones and PPARgamma agonists: time for a reassessment. Trends Endocrinol Metab 23: 205–215. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical