Identification of the immunodominant regions of Staphylococcus aureus fibronectin-binding protein A
- PMID: 24736634
- PMCID: PMC3988184
- DOI: 10.1371/journal.pone.0095338
Identification of the immunodominant regions of Staphylococcus aureus fibronectin-binding protein A
Abstract
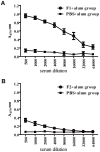
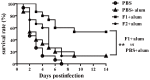
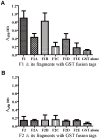
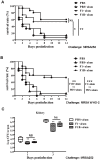
Staphylococcus aureus is an opportunistic bacterial pathogen responsible for a diverse spectrum of human diseases and a leading cause of nosocomial and community-acquired infections. Development of a vaccine against this pathogen is an important goal. The fibronectin binding protein A (FnBPA) of S. aureus is one of multifunctional 'microbial surface components recognizing adhesive matrix molecules' (MSCRAMMs). It is one of the most important adhesin molecules involved in the initial adhesion steps of S. aureus infection. It has been studied as potential vaccine candidates. However, FnBPA is a high-molecular-weight protein of 106 kDa and difficulties in achieving its high-level expression in vitro limit its vaccine application in S. aureus infection diseases control. Therefore, mapping the immunodominant regions of FnBPA is important for developing polyvalent subunit fusion vaccines against S. aureus infections. In the present study, we cloned and expressed the N-terminal and C-terminal of FnBPA. We evaluated the immunogenicity of the two sections of FnBPA and the protective efficacy of the two truncated fragments vaccines in a murine model of systemic S. aureus infection. The results showed recombinant truncated fragment F130-500 had a strong immunogenicity property and survival rates significantly increased in the group of mice immunized with F130-500 than the control group. We futher identified the immunodominant regions of FnBPA. The mouse antisera reactions suggest that the region covering residues 110 to 263 (F1B110-263) is highly immunogenic and is the immunodominant regions of FnBPA. Moreover, vaccination with F1B110-263 can generate partial protection against lethal challenge with two different S. aureus strains and reduced bacterial burdens against non-lethal challenge as well as that immunization with F130-500. This information will be important for further developing anti- S. aureus polyvalent subunit fusion vaccines.
Conflict of interest statement
Figures


References
-
- Taccetti G, Cocchi P, Festini F, Braggion C, Campana S (2010) Community-associated meticillin-resistant Staphylococcus aureus. Lancet 376: 767–768. - PubMed
-
- Durai R, Ng PC, Hoque H (2010) Methicillin-resistant Staphylococcus aureus: an update. AORN J 91: : 599–606; quiz 607–599. - PubMed
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, et al. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298: 1763–1771. - PubMed
-
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, et al. (2001) Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 32 Suppl 2: S114–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

