Alveolar bone loss: mechanisms, potential therapeutic targets, and interventions
- PMID: 24736703
- PMCID: PMC6636231
- DOI: 10.1177/0022034514529305
Alveolar bone loss: mechanisms, potential therapeutic targets, and interventions
Abstract
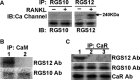
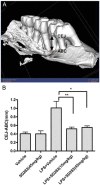
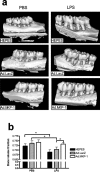
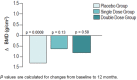
This article reviews recent research into mechanisms underlying bone resorption and highlights avenues of investigation that may generate new therapies to combat alveolar bone loss in periodontitis. Several proteins, signaling pathways, stem cells, and dietary supplements are discussed as they relate to periodontal bone loss and regeneration. RGS12 is a crucial protein that mediates osteoclastogenesis and bone destruction, and a potential therapeutic target. RGS12 likely regulates osteoclast differentiation through regulating calcium influx to control the calcium oscillation-NFATc1 pathway. A working model for RGS10 and RGS12 in the regulation of Ca(2+) oscillations during osteoclast differentiation is proposed. Initiation of inflammation depends on host cell-microbe interactions, including the p38 mitogen-activated protein kinase (MAPK) signaling pathway. Oral p38 inhibitors reduced lipopolysaccharide (LPS)-induced bone destruction in a rat periodontitis model but showed unsatisfactory safety profiles. The p38 substrate MK2 is a more specific therapeutic target with potentially superior tolerability. Furthermore, MKP-1 shows anti-inflammatory activity, reducing inflammatory cytokine biosynthesis and bone resorption. Multipotent skeletal stem cell (SSC) populations exist within the bone marrow and periosteum of long bones. These bone-marrow-derived SSCs and periosteum-derived SSCs have shown therapeutic potential in several applications, including bone and periodontal regeneration. The existence of craniofacial bone-specific SSCs is suggested based on existing studies. The effects of calcium, vitamin D, and soy isoflavone supplementation on alveolar and skeletal bone loss in post-menopausal women were investigated. Supplementation resulted in stabilization of forearm bone mass density and a reduced rate of alveolar bone loss over 1 yr, compared with placebo. Periodontal attachment levels were also well-maintained and alveolar bone loss suppressed during 24 wk of supplementation.
Keywords: menopause; mesenchymal stem cells; p38 MAPK; p38 inhibitors; regulator of G protein signaling; skeletal stem cells.
Figures

Similar articles
-
A p38 mitogen-activated protein kinase inhibitor arrests active alveolar bone loss in a rat periodontitis model.J Periodontol. 2007 Oct;78(10):1992-8. doi: 10.1902/jop.2007.070101. J Periodontol. 2007. PMID: 18062121
-
Effect of a mixture of calcium, vitamin D, inulin and soy isoflavones on bone metabolism in post-menopausal women: a retrospective analysis.Aging Clin Exp Res. 2013 Dec;25(6):611-7. doi: 10.1007/s40520-013-0093-y. Epub 2013 Aug 2. Aging Clin Exp Res. 2013. PMID: 23907774
-
A novel Bruton's tyrosine kinase inhibitor, acalabrutinib, suppresses osteoclast differentiation and Porphyromonas gingivalis lipopolysaccharide-induced alveolar bone resorption.J Periodontol. 2019 May;90(5):546-554. doi: 10.1002/JPER.18-0334. Epub 2018 Nov 20. J Periodontol. 2019. PMID: 30387495
-
Vitamin D interactions with soy isoflavones on bone after menopause: a review.Nutrients. 2012 Nov 6;4(11):1610-21. doi: 10.3390/nu4111610. Nutrients. 2012. PMID: 23201836 Free PMC article. Review.
-
Immune response: the key to bone resorption in periodontal disease.J Periodontol. 2005 Nov;76(11 Suppl):2033-41. doi: 10.1902/jop.2005.76.11-S.2033. J Periodontol. 2005. PMID: 16277573 Review.
Cited by
-
Association between chronic obstructive pulmonary disease and periodontal disease: a systematic review and meta-analysis.BMJ Open. 2023 Jun 26;13(6):e067432. doi: 10.1136/bmjopen-2022-067432. BMJ Open. 2023. PMID: 37369414 Free PMC article.
-
Electrospun Nanofibers for Periodontal Treatment: A Recent Progress.Int J Nanomedicine. 2022 Sep 12;17:4137-4162. doi: 10.2147/IJN.S370340. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36118177 Free PMC article. Review.
-
Combination adipose-derived mesenchymal stem cells-demineralized dentin matrix increase bone marker expression in periodontitis rats.Saudi Dent J. 2023 Dec;35(8):960-968. doi: 10.1016/j.sdentj.2023.07.019. Epub 2023 Aug 2. Saudi Dent J. 2023. PMID: 38107047 Free PMC article.
-
N-Acetyl-l-Leucine-Polyethyleneimine-Mediated Delivery of CpG Oligodeoxynucleotides 2006 Inhibits RAW264.7 Cell Osteoclastogenesis.Drug Des Devel Ther. 2020 Jul 7;14:2657-2665. doi: 10.2147/DDDT.S241826. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32764870 Free PMC article.
-
The Potential of Different Origin Stem Cells in Modulating Oral Bone Regeneration Processes.Cells. 2019 Jan 8;8(1):29. doi: 10.3390/cells8010029. Cells. 2019. PMID: 30625993 Free PMC article. Review.
References
-
- Allen MR, Hock JM, Burr DB. (2004). Periosteum: biology, regulation, and response to osteoporosis therapies. Bone 35: 1003–1012. - PubMed
-
- Baker PJ, Howe L, Garneau J, Roopenian DC. (2002). T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol Med Microbiol 34: 45–50. - PubMed
-
- Bhattacherjee V, Mukhopadhyay P, Singh S, Johnson C, Philipose JT, Warner CP, et al. (2007). Neural crest and mesoderm lineage-dependent gene expression in orofacial development. Differentiation 75: 463–477. - PubMed
-
- Bianco P, Riminucci M, Gronthos S, Robey PG. (2001). Bone marrow stro-mal stem cells: nature, biology, and potential applications. Stem cells 19: 180–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR055678/AR/NIAMS NIH HHS/United States
- K08 AR055678/AR/NIAMS NIH HHS/United States
- K99 DE021069/DE/NIDCR NIH HHS/United States
- P30 GM103331/GM/NIGMS NIH HHS/United States
- R01 AG048388/AG/NIA NIH HHS/United States
- R03 DE016857/DE/NIDCR NIH HHS/United States
- R00 DE021069/DE/NIDCR NIH HHS/United States
- DE016857/DE/NIDCR NIH HHS/United States
- R01 AR066101/AR/NIAMS NIH HHS/United States
- R01 DE018290/DE/NIDCR NIH HHS/United States
- R01DE018290/DE/NIDCR NIH HHS/United States
- P30GM103331/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

