Activated macrophage survival is coordinated by TAK1 binding proteins
- PMID: 24736749
- PMCID: PMC3988229
- DOI: 10.1371/journal.pone.0094982
Activated macrophage survival is coordinated by TAK1 binding proteins
Abstract
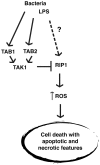
Macrophages play diverse roles in tissue homeostasis and immunity, and canonically activated macrophages are critically associated with acute inflammatory responses. It is known that activated macrophages undergo cell death after transient activation in some settings, and the viability of macrophages impacts on inflammatory status. Here we report that TGFβ- activated kinase (TAK1) activators, TAK1-binding protein 1 (TAB1) and TAK1-binding protein 2 (TAB2), are critical molecules in the regulation of activated macrophage survival. While deletion of Tak1 induced cell death in bone marrow derived macrophages even without activation, Tab1 or Tab2 deletion alone did not profoundly affect survival of naïve macrophages. However, in lipopolysaccharide (LPS)-activated macrophages, even single deletion of Tab1 or Tab2 resulted in macrophage death with both necrotic and apoptotic features. We show that TAB1 and TAB2 were redundantly involved in LPS-induced TAK1 activation in macrophages. These results demonstrate that TAK1 activity is the key to activated macrophage survival. Finally, in an in vivo setting, Tab1 deficiency impaired increase of peritoneal macrophages upon LPS challenge, suggesting that TAK1 complex regulation of macrophages may participate in in vivo macrophage homeostasis. Our results demonstrate that TAB1 and TAB2 are required for activated macrophages, making TAB1 and TAB2 effective targets to control inflammation by modulating macrophage survival.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

