Effect of caffeine on SPECT myocardial perfusion imaging during regadenoson pharmacologic stress: a prospective, randomized, multicenter study
- PMID: 24737255
- PMCID: PMC4008779
- DOI: 10.1007/s10554-014-0419-7
Effect of caffeine on SPECT myocardial perfusion imaging during regadenoson pharmacologic stress: a prospective, randomized, multicenter study
Abstract
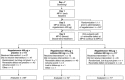
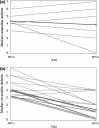
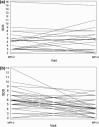
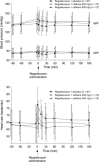
A multicenter, double-blind, randomized study was conducted to assess the effect of caffeine on regadenoson stress myocardial perfusion imaging (MPI). Subjects with a high likelihood of coronary artery disease underwent a rest single-photon emission computed tomography MPI on day 1 (MPI-1) and a stress MPI with regadenoson on day 3 (MPI-2). Individuals with ≥1 segment with a reversible defect received double-blind caffeine tablets (200 or 400 mg) or placebo 90 min before a repeat regadenoson stress MPI (MPI-3) on day 5. Overall, 207 subjects completed the study (caffeine 200 mg, n = 70; caffeine 400 mg, n = 71; placebo, n = 66). The mean number of segments with reversible defects decreased from MPI-2 to MPI-3 in the caffeine 200 and 400 mg groups versus no significant change in the placebo group [mean ± standard deviation: -0.61 ± 1.097, -0.62 ± 1.367, and 0.12 ± 0.981, respectively (overall treatment effect, P < 0.001)]. The majority of subjects who received caffeine shifted to a lower ischemia size category from MPI-2 to MPI-3, with no clear pattern observed in subjects who received placebo. For caffeine exposed patients with ≥3 segments with reversible defects at MPI-2, 21/23 had fewer detected at MPI-3. Both the 200 and 400 mg doses of caffeine significantly reduced the number of segments with reversible defects detected by regadenoson stress MPI.
Figures


Similar articles
-
Effect of caffeine on SPECT myocardial perfusion imaging during regadenoson pharmacologic stress: rationale and design of a prospective, randomized, multicenter study.J Nucl Cardiol. 2011 Feb;18(1):73-81. doi: 10.1007/s12350-010-9311-6. Epub 2010 Nov 17. J Nucl Cardiol. 2011. PMID: 21082298 Clinical Trial.
-
Regadenoson provides perfusion results comparable to adenosine in heterogeneous patient populations: a quantitative analysis from the ADVANCE MPI trials.J Nucl Cardiol. 2015 Apr;22(2):248-61. doi: 10.1007/s12350-014-9981-6. Epub 2014 Oct 7. J Nucl Cardiol. 2015. PMID: 25287737 Clinical Trial.
-
Prognostic value of regadenoson stress myocardial perfusion imaging in patients with left bundle branch block or ventricular paced rhythm.J Nucl Cardiol. 2021 Jun;28(3):967-977. doi: 10.1007/s12350-019-01762-4. Epub 2019 May 29. J Nucl Cardiol. 2021. PMID: 31144225
-
Regadenoson use in chronic kidney disease and end-stage renal disease: A focused review.J Nucl Cardiol. 2018 Feb;25(1):137-149. doi: 10.1007/s12350-017-0960-6. Epub 2017 Jun 26. J Nucl Cardiol. 2018. PMID: 28653271 Review.
-
Regadenoson: a focused update.J Nucl Cardiol. 2013 Apr;20(2):284-8. doi: 10.1007/s12350-012-9661-3. J Nucl Cardiol. 2013. PMID: 23229649 Review.
Cited by
-
Guidelines for Cardiovascular Magnetic Resonance Imaging from the Korean Society of Cardiovascular Imaging-Part 3: Perfusion, Delayed Enhancement, and T1- and T2 Mapping.Korean J Radiol. 2019 Dec;20(12):1562-1582. doi: 10.3348/kjr.2019.0411. Korean J Radiol. 2019. PMID: 31854146 Free PMC article. Review.
-
ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers.J Nucl Cardiol. 2016 Jun;23(3):606-39. doi: 10.1007/s12350-015-0387-x. J Nucl Cardiol. 2016. PMID: 26914678 No abstract available.
-
Caffeine does not significantly reduce the sensitivity of vasodilator stress MPI: Rebuttal.J Nucl Cardiol. 2016 Jun;23(3):604. doi: 10.1007/s12350-016-0421-7. Epub 2016 Feb 10. J Nucl Cardiol. 2016. PMID: 26864091 No abstract available.
-
Quantitative myocardial perfusion response to adenosine and regadenoson in patients with suspected coronary artery disease.J Nucl Cardiol. 2022 Feb;29(1):24-36. doi: 10.1007/s12350-021-02731-6. Epub 2021 Aug 12. J Nucl Cardiol. 2022. PMID: 34386859 Free PMC article.
-
Caffeine Consumption and Heart Rate and Blood Pressure Response to Regadenoson.PLoS One. 2015 Jun 22;10(6):e0130487. doi: 10.1371/journal.pone.0130487. eCollection 2015. PLoS One. 2015. PMID: 26098883 Free PMC article.
References
-
- Astellas Pharma US Inc. (2012) Lexiscan® (regadenoson) injection for intravenous administration. Full prescribing information. http://www.astellas.us/docs/lexiscan.pdf. Accessed 19 Aug 2013
-
- Ananthasubramaniam K, Weiss R, McNutt B, Klauke B, Feaheny K, Bukofzer S. A randomized, double-blind, placebo-controlled study of the safety and tolerance of regadenoson in subjects with stage 3 or 4 chronic kidney disease. J Nucl Cardiol. 2012;19(2):319–329. doi: 10.1007/s12350-011-9508-3. - DOI - PMC - PubMed
-
- Prenner BM, Bukofzer S, Behm S, Feaheny K, McNutt BE. A randomized, double-blind, placebo-controlled study assessing the safety and tolerability of regadenoson in subjects with asthma or chronic obstructive pulmonary disease. J Nucl Cardiol. 2012;19(4):681–692. doi: 10.1007/s12350-012-9547-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

