Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study
- PMID: 24737258
- PMCID: PMC4028549
- DOI: 10.1007/s00134-014-3278-8
Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study
Abstract
Purpose: Ulinastatin, a serine protease inhibitor, inhibits several pro-inflammatory proteases and decreases inflammatory cytokine levels and mortality in experimental sepsis. We studied the effect of ulinastatin on 28-day all-cause mortality in a double-blind trial in patients with severe sepsis in seven Indian hospitals.
Methods: Patients with sepsis were randomized within 48 h of onset of one or more organ failures to receive intravenous administration of ulinastatin (200,000 IU) or placebo 12 hourly for 5 days.
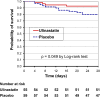
Results: Of 122 randomized subjects, 114 completed the study (55 receiving ulinastatin, 59 receiving placebo). At baseline, the mean APACHE II score was 13.4 (SD = 4.4), 48 (42 %) patients were receiving mechanical ventilation, 58 (51 %) were on vasopressors, and 35 % had multiple organ failure. In the modified intention-to-treat analysis (patients receiving six or more doses of study drugs), 28-day all-cause mortality was 7.3 % with ulinastatin (4 deaths) versus 20.3 % (12 deaths) with placebo (p = 0.045). On multivariate analysis too, treatment with ulinastatin (odds ratio 0.26, 95 % CI 0.07-0.95; p = 0.042) independently decreased 28-day all-cause mortality. However, the mortality difference did not reach statistical significance in the intention-to-treat analysis [10.2 % (6/59 deaths) with ulinastatin versus 20.6 % (13/63 deaths) in the placebo group; p = 0.11]. The ulinastatin group had lower incidence of new-onset organ failure (10 vs. 26 patients, p = 0.003), more ventilator-free days (mean ± SD 19.4 ± 10.6 days vs. 10.2 ± 12.5 days, p = 0.019), and shorter hospital stay (11.8 ± 7.1 days vs. 24.2 ± 7.2 days, p < 0.001).
Conclusions: In this pilot study, intravenous administration of ulinastatin reduced mortality in patients with severe sepsis in the modified intention-to-treat analysis, but not in the intention-to-treat analysis.
Figures
Comment in
-
Ulinastatin: is it worth using in severe sepsis?Intensive Care Med. 2014 Aug;40(8):1185. doi: 10.1007/s00134-014-3341-5. Epub 2014 May 21. Intensive Care Med. 2014. PMID: 24845579 No abstract available.
-
An exciting candidate therapy for sepsis: ulinastatin, a urinary protease inhibitor.Intensive Care Med. 2014 Aug;40(8):1164-7. doi: 10.1007/s00134-014-3366-9. Epub 2014 Jul 3. Intensive Care Med. 2014. PMID: 24990495 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical