Reciprocal changes in phosphorylation and methylation of mammalian brain sodium channels in response to seizures
- PMID: 24737319
- PMCID: PMC4140893
- DOI: 10.1074/jbc.M114.562785
Reciprocal changes in phosphorylation and methylation of mammalian brain sodium channels in response to seizures
Abstract
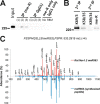
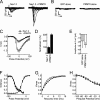
Voltage-gated sodium (Nav) channels initiate action potentials in brain neurons and are primary therapeutic targets for anti-epileptic drugs controlling neuronal hyperexcitability in epilepsy. The molecular mechanisms underlying abnormal Nav channel expression, localization, and function during development of epilepsy are poorly understood but can potentially result from altered posttranslational modifications (PTMs). For example, phosphorylation regulates Nav channel gating, and has been proposed to contribute to acquired insensitivity to anti-epileptic drugs exhibited by Nav channels in epileptic neurons. However, whether changes in specific brain Nav channel PTMs occur acutely in response to seizures has not been established. Here, we show changes in PTMs of the major brain Nav channel, Nav1.2, after acute kainate-induced seizures. Mass spectrometry-based proteomic analyses of Nav1.2 purified from the brains of control and seizure animals revealed a significant down-regulation of phosphorylation at nine sites, primarily located in the interdomain I-II linker, the region of Nav1.2 crucial for phosphorylation-dependent regulation of activity. Interestingly, Nav1.2 in the seizure samples contained methylated arginine (MeArg) at three sites. These MeArgs were adjacent to down-regulated sites of phosphorylation, and Nav1.2 methylation increased after seizure. Phosphorylation and MeArg were not found together on the same tryptic peptide, suggesting reciprocal regulation of these two PTMs. Coexpression of Nav1.2 with the primary brain arginine methyltransferase PRMT8 led to a surprising 3-fold increase in Nav1.2 current. Reciprocal regulation of phosphorylation and MeArg of Nav1.2 may underlie changes in neuronal Nav channel function in response to seizures and also contribute to physiological modulation of neuronal excitability.
Keywords: Electrophysiology; Epilepsy; Ion Channels; Mass Spectrometry (MS); Proteomics.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Catterall W. A., Goldin A. L., Waxman S. G. (2005) International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 57, 397–409 - PubMed
-
- Cantrell A. R., Catterall W. A. (2001) Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat. Rev. Neurosci. 2, 397–407 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

