A general principle governs vision-dependent dendritic patterning of retinal ganglion cells
- PMID: 24737624
- PMCID: PMC4139438
- DOI: 10.1002/cne.23609
A general principle governs vision-dependent dendritic patterning of retinal ganglion cells
Abstract
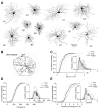
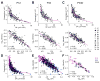
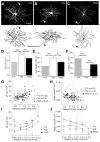
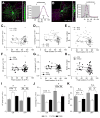
Dendritic arbors of retinal ganglion cells (RGCs) collect information over a certain area of the visual scene. The coverage territory and the arbor density of dendrites determine what fraction of the visual field is sampled by a single cell and at what resolution. However, it is not clear whether visual stimulation is required for the establishment of branching patterns of RGCs, and whether a general principle directs the dendritic patterning of diverse RGCs. By analyzing the geometric structures of RGC dendrites, we found that dendritic arbors of RGCs underwent a substantial spatial rearrangement after eye-opening. Light deprivation blocked both the dendritic growth and the branch patterning, suggesting that visual stimulation is required for the acquisition of specific branching patterns of RGCs. We further showed that vision-dependent dendritic growth and arbor refinement occurred mainly in the middle portion of the dendritic tree. This nonproportional growth and selective refinement suggest that the late-stage dendritic development of RGCs is not a passive stretching with the growth of eyes, but rather an active process of selective growth/elimination of dendritic arbors of RGCs driven by visual activity. Finally, our data showed that there was a power law relationship between the coverage territory and dendritic arbor density of RGCs on a cell-by-cell basis. RGCs were systematically less dense when they cover larger territories regardless of their cell type, retinal location, or developmental stage. These results suggest that a general structural design principle directs the vision-dependent patterning of RGC dendrites.
Keywords: dendritic density and coverage territory; dendritic development; retinal ganglion cell; visual activity.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
Figures



Similar articles
-
Cellular and Molecular Analysis of Dendritic Morphogenesis in a Retinal Cell Type That Senses Color Contrast and Ventral Motion.J Neurosci. 2017 Dec 13;37(50):12247-12262. doi: 10.1523/JNEUROSCI.2098-17.2017. Epub 2017 Nov 7. J Neurosci. 2017. PMID: 29114073 Free PMC article.
-
Factors controlling the dendritic arborization of retinal ganglion cells.Vis Neurosci. 1996 Jul-Aug;13(4):721-33. doi: 10.1017/s0952523800008609. Vis Neurosci. 1996. PMID: 8870228
-
Retinal ganglion cell dendrites undergo a visual activity-dependent redistribution after eye opening.J Comp Neurol. 2007 Jul 10;503(2):244-59. doi: 10.1002/cne.21379. J Comp Neurol. 2007. PMID: 17492624
-
Pathway-specific maturation, visual deprivation, and development of retinal pathway.Neuroscientist. 2004 Aug;10(4):337-46. doi: 10.1177/1073858404265254. Neuroscientist. 2004. PMID: 15271261 Review.
-
The role of early neural activity in the maturation of turtle retinal function.J Anat. 2001 Oct;199(Pt 4):375-83. doi: 10.1046/j.1469-7580.2001.19940375.x. J Anat. 2001. PMID: 11693298 Free PMC article. Review.
Cited by
-
Are visual peripheries forever young?Neural Plast. 2015;2015:307929. doi: 10.1155/2015/307929. Epub 2015 Apr 6. Neural Plast. 2015. PMID: 25945262 Free PMC article. Review.
-
The effects of immune protein CD3ζ development and degeneration of retinal neurons after optic nerve injury.PLoS One. 2017 Apr 25;12(4):e0175522. doi: 10.1371/journal.pone.0175522. eCollection 2017. PLoS One. 2017. PMID: 28441398 Free PMC article.
-
Role for Visual Experience in the Development of Direction-Selective Circuits.Curr Biol. 2016 May 23;26(10):1367-75. doi: 10.1016/j.cub.2016.03.073. Epub 2016 May 5. Curr Biol. 2016. PMID: 27161499 Free PMC article.
-
Spatial registration of neuron morphologies based on maximization of volume overlap.BMC Bioinformatics. 2018 Apr 18;19(1):143. doi: 10.1186/s12859-018-2136-z. BMC Bioinformatics. 2018. PMID: 29669537 Free PMC article.
References
-
-
Antibodies: “YFP was localized using a rabbit polyclonal antibody against GFP isolated directly from Aequorea Victoria (Molecular Probes, Cat# A21311, RRID: AB_10058149)” and “Tyrosine hydroxylase was localized using a sheep polyclonal antibody against pheochromocytoma Tyrosine hydroxylase (Chemicon Cat# AB1542, RRID: AB_90755)”
-
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. - PMC - PubMed
-
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

