Comparative anatomy of floral elaiophores in Vitekorchis Romowicz & Szlach., Cyrtochilum Kunth and a florally dimorphic species of Oncidium Sw. (Orchidaceae: Oncidiinae)
- PMID: 24737719
- PMCID: PMC4030811
- DOI: 10.1093/aob/mcu045
Comparative anatomy of floral elaiophores in Vitekorchis Romowicz & Szlach., Cyrtochilum Kunth and a florally dimorphic species of Oncidium Sw. (Orchidaceae: Oncidiinae)
Abstract
Background and aims: Recently, molecular approaches have been used to investigate the phylogeny of subtribe Oncidiinae, resulting in the re-alignment of several of its genera. Here, a description is given of the structure of the floral elaiophores (oil glands) of four species formerly assigned to Oncidium Sw. Those of Vitekorchis excavata (Lindl.) Romowicz & Szlach., Cyrtochilum meirax (Rchb.f.) Dalström and a species of Oncidium displaying floral dimorphism, namely O. heteranthum Poepp. & Endl. var. album, are compared with that of Gomesa longipes (Lindl.) M.W. Chase & N.H. Williams, whose epithelial elaiophores are typical of many Oncidiinae, in order to extend our understanding of elaiophore diversity within this subtribe.
Methods: Floral elaiophore structure was examined and compared at anthesis for all four species using light microscopy, scanning electron microscopy, transmission electron microscopy and histochemistry.
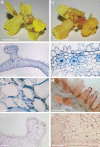
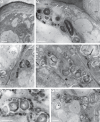
Key results: In all species investigated, with the exception of C. meirax, the floral elaiophore occurs on the labellar callus and is of the intermediate type, possessing both glabrous and trichomatous regions. By contrast, although all four species produce lipid secretions, C. meirax lacks an obvious elaiophore. In each case, the secretory tissue is represented by a single-layered epidermis of cuboidal cells (trichomatous and/or atrichomatous). Palisade cells are absent. The secretion may be wax- or oil-like and is usually produced by smooth endoplasmic reticulum (SER). However, in C. meirax, where rough endoplasmic reticulum (RER) predominates, oil accumulates as plastoglobuli within elaioplasts. These plastoglobuli are then discharged into the cytoplasm, forming oil bodies. In some species, oil usually accumulates within vesicles at the plasmalemma or in the periplasmic space before traversing the cell wall and accumulating beneath the cuticle, sometimes with distension of the latter. Gomesa longipes is unusual in its production of a heterogeneous secretion, whereas Vitekorchis excavata is equally remarkable for the protuberances found on the walls of its secretory cells.
Conclusions: Anatomically, the secretory tissues of all four species, despite currently being assigned to four different genera, are remarkably similar and indicative of homoplasy. This supports previous investigations of the floral elaiophore in Oncidiinae, which showed that the same elaiophore characters may be shared by different clades, but not always by species of the same genus. Consequently, elaiophores are considered to be of limited value in investigating the phylogeny of this subtribe. Furthermore, floral dimorphism does not greatly modify elaiophore structure in the fertile flowers of Oncidium heteranthum var. album. Based on the presence or absence of well-defined elaiophores, the nature of the secretion and the cell ultrastructure, it is likely that floral oil may be produced in Oncidiinae in one of two ways: by the ER (mainly SER) or by plastids, most notably elaioplasts. Once the oil is discharged into the cytoplasm as oil bodies or oil droplets, there is little difference between the subsequent stages of oil secretion; the oil traversing the cytoplasm (often vesicle-mediated) and cell wall before accumulating beneath the cuticle.
Keywords: Anatomy; Cyrtochilum; Gomesa; Oncidiinae; Oncidium; Orchidaceae; Vitekorchis; floral elaiophore; lipids; micromorphology; oil glands; ultrastructure.
© The Author 2014. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures










Similar articles
-
Comparative anatomy of the floral elaiophore in representatives of the newly re-circumscribed Gomesa and Oncidium clades (Orchidaceae: Oncidiinae).Ann Bot. 2013 Sep;112(5):839-54. doi: 10.1093/aob/mct149. Epub 2013 Jul 24. Ann Bot. 2013. PMID: 23884394 Free PMC article.
-
Floral elaiophore structure in four representatives of the Ornithocephalus clade (Orchidaceae: Oncidiinae).Ann Bot. 2012 Sep;110(4):809-20. doi: 10.1093/aob/mcs158. Epub 2012 Jul 17. Ann Bot. 2012. PMID: 22805528 Free PMC article.
-
Comparative histology of floral elaiophores in the orchids Rudolfiella picta (Schltr.) Hoehne (Maxillariinae sensu lato) and Oncidium ornithorhynchum H.B.K. (Oncidiinae sensu lato).Ann Bot. 2009 Aug;104(2):221-34. doi: 10.1093/aob/mcp119. Epub 2009 May 15. Ann Bot. 2009. PMID: 19447811 Free PMC article.
-
Development and evolution of extreme synorganization in angiosperm flowers and diversity: a comparison of Apocynaceae and Orchidaceae.Ann Bot. 2016 Apr;117(5):749-67. doi: 10.1093/aob/mcv119. Epub 2015 Aug 20. Ann Bot. 2016. PMID: 26292994 Free PMC article. Review.
-
An overview of secretion in floral bracts of Tillandsioideae (Bromeliaceae), with emphasis on the secretory scales.AoB Plants. 2023 Sep 26;15(5):plad066. doi: 10.1093/aobpla/plad066. eCollection 2023 Oct. AoB Plants. 2023. PMID: 37899979 Free PMC article. Review.
Cited by
-
Pollination of Specklinia by nectar-feeding Drosophila: the first reported case of a deceptive syndrome employing aggregation pheromones in Orchidaceae.Ann Bot. 2015 Sep;116(3):437-55. doi: 10.1093/aob/mcv086. Epub 2015 Jun 13. Ann Bot. 2015. PMID: 26071932 Free PMC article.
-
Comparative survey of secretory structures and floral anatomy of Cohniella cepula and Cohniella jonesiana (Orchidaceae: Oncidiinae). New evidences of nectaries and osmophores in the genus.Protoplasma. 2019 May;256(3):703-720. doi: 10.1007/s00709-018-1330-1. Epub 2018 Nov 23. Protoplasma. 2019. PMID: 30470901
-
Floral glands in myophilous and sapromyophilous species of Pleurothallidinae (Epidendroideae, Orchidaceae)-osmophores, nectaries, and a unique sticky gland.Protoplasma. 2021 Sep;258(5):1061-1076. doi: 10.1007/s00709-021-01624-2. Epub 2021 Feb 22. Protoplasma. 2021. PMID: 33619653
-
Floral ultrastructure of two Brazilian aquatic-epiphytic bladderworts: Utricularia cornigera Studnička and U. nelumbifolia Gardner (Lentibulariaceae).Protoplasma. 2017 Jan;254(1):353-366. doi: 10.1007/s00709-016-0956-0. Epub 2016 Mar 5. Protoplasma. 2017. PMID: 26945989 Free PMC article.
References
-
- Brummitt RK, Powell CE. Authors of plant names. Kew, UK: Royal Botanic Gardens; 1992.
-
- Buchmann SL. The ecology of oil flowers and their bees. Annual Review of Ecology and Systematics. 1987;18:343–369.
-
- Chase MW. Classification of Orchidaceae in the age of DNA data. Curtis's Botanical Magazine. 2005;22:2–7.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

