Microscale technologies for regulating human stem cell differentiation
- PMID: 24737735
- PMCID: PMC4476254
- DOI: 10.1177/1535370214530369
Microscale technologies for regulating human stem cell differentiation
Abstract
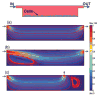
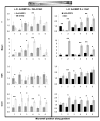
During development and regeneration, tissues emerge from coordinated sequences of stem cell renewal, specialization, and assembly that are orchestrated by cascades of regulatory factors. This complex in vivo milieu, while necessary to fully recapitulate biology and to properly engineer progenitor cells, is difficult to replicate in vitro. We are just starting to fully realize the importance of the entire context of cell microenvironment-the other cells, three-dimensional matrix, molecular and physical signals. Bioengineered environments that combine tissue-specific transport and signaling are critical to study cellular responses at biologically relevant scales and in settings predictive of human condition. We therefore developed microbioreactors that couple the application of fast dynamic changes in environmental signals with versatile, high-throughput operation and imaging capability. Our base device is a microfluidic platform with an array of microwells containing cells or tissue constructs that are exposed to stable concentration gradients. Mathematical modeling of flow and mass transport can predict the shape of these gradients and the kinetic changes in local concentrations. A single platform, the size of a microscope slide, contains up to 120 biological samples. As an example of application, we describe studies of cell fate specification and mesodermal lineage commitment in human embryonic stem cells and induced pluripotent stem cells. The embryoid bodies formed from these cells were subjected to single and multiple concentration gradients of Wnt3a, Activin A, bone morphogenic protein 4 (BMP4), and their inhibitors, and the gene expression profiles were correlated to the concentration gradients of morphogens to identify the exact conditions for mesodermal differentiation.
Keywords: Human stem cells; cardiac differentiation; flow; gradients; microscale platforms; transport.
© 2014 by the Society for Experimental Biology and Medicine.
Figures




Similar articles
-
Microfluidic bioreactor for dynamic regulation of early mesodermal commitment in human pluripotent stem cells.Lab Chip. 2013 Feb 7;13(3):355-64. doi: 10.1039/c2lc40836h. Lab Chip. 2013. PMID: 23232509 Free PMC article.
-
Confined 3D microenvironment regulates early differentiation in human pluripotent stem cells.Biotechnol Bioeng. 2012 Dec;109(12):3119-32. doi: 10.1002/bit.24571. Epub 2012 Jun 20. Biotechnol Bioeng. 2012. PMID: 22674472
-
Microfluidic device generating stable concentration gradients for long term cell culture: application to Wnt3a regulation of β-catenin signaling.Lab Chip. 2010 Dec 7;10(23):3277-83. doi: 10.1039/c0lc00033g. Epub 2010 Oct 11. Lab Chip. 2010. PMID: 20936235 Free PMC article.
-
Engineering approaches toward deconstructing and controlling the stem cell environment.Ann Biomed Eng. 2012 Jun;40(6):1301-15. doi: 10.1007/s10439-011-0452-9. Epub 2011 Nov 19. Ann Biomed Eng. 2012. PMID: 22101755 Free PMC article. Review.
-
Current Technologies Based on the Knowledge of the Stem Cells Microenvironments.Adv Exp Med Biol. 2017;1041:245-262. doi: 10.1007/978-3-319-69194-7_13. Adv Exp Med Biol. 2017. PMID: 29204837 Review.
Cited by
-
The relevance and potential roles of microphysiological systems in biology and medicine.Exp Biol Med (Maywood). 2014 Sep;239(9):1061-72. doi: 10.1177/1535370214542068. Exp Biol Med (Maywood). 2014. PMID: 25187571 Free PMC article.
-
Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology.Exp Biol Med (Maywood). 2017 Oct;242(16):1559-1572. doi: 10.1177/1535370217732765. Exp Biol Med (Maywood). 2017. PMID: 29065799 Free PMC article.
-
Spatially controlled stem cell differentiation via morphogen gradients: A comparison of static and dynamic microfluidic platforms.J Vac Sci Technol A. 2020 May;38(3):033205. doi: 10.1116/1.5142012. Epub 2020 Mar 24. J Vac Sci Technol A. 2020. PMID: 32255900 Free PMC article.
-
Engineered Biomaterials to Enhance Stem Cell-Based Cardiac Tissue Engineering and Therapy.Macromol Biosci. 2016 Jul;16(7):958-77. doi: 10.1002/mabi.201500396. Epub 2016 Mar 8. Macromol Biosci. 2016. PMID: 26953627 Free PMC article.
-
Biology coming full circle: joining the whole and the parts.Exp Biol Med (Maywood). 2015 Jan;240(1):3-7. doi: 10.1177/1535370214564534. Exp Biol Med (Maywood). 2015. PMID: 25583953 Free PMC article.
References
-
- Kaplan D, Moon RT, Vunjak-Novakovic G. It takes a village to grow a tissue. Nat Biotechnol. 2005;23:1237–9. - PubMed
-
- Powell K. Stem-cell niches: It’s the ecology, stupid! Nature. 2005;435:268–70. - PubMed
-
- Metallo CM, Mohr JC, Detzel CJ, de Pablo JJ, Van Wie BJ, Palecek SP. Engineering the stem cell microenvironment. Biotechnol Prog. 2007;23:18–23. - PubMed
-
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–80. - PubMed
-
- Zhang S. Beyond the petri dish. Nat Biotechnol. 2004;22:151–2. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

