Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming
- PMID: 24737868
- PMCID: PMC4193918
- DOI: 10.1002/embj.201488027
Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming
Abstract
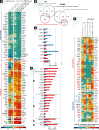
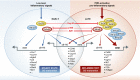
Dendritic cells (DC) are key regulators of both protective immune responses and tolerance to self-antigens. Soon after their discovery in lymphoid tissues by Steinman and Cohn, as cells with the unique ability to prime naïve antigen-specific T cells, it was realized that DC can exist in at least two distinctive states characterized by morphological, phenotypic and functional changes-this led to the description of DC maturation. It is now well appreciated that there are several subsets of DC in both lymphoid and non-lymphoid tissues of mammals, and these cells show remarkable functional specialization and specificity in their roles in tolerance and immunity. This review will focus on the specific characteristics of DC subsets and how their functional specialization may be regulated by distinctive gene expression programs and signaling responses in both steady-state and in the context of inflammation. In particular, we will highlight the common and distinctive genes and signaling pathways that are associated with the functional maturation of DC subsets.
Keywords: dendritic cells; homeostasis; immunity; tolerance.
© 2014 The Authors.
Figures


References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. - PubMed
-
- Andrade WA, Souza Mdo C, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, Bartholomeu DC, Ghosh S, Golenbock DT, Gazzinelli RT. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe. 2013;13:42–53. - PMC - PubMed
-
- Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O'Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

