Monocyte-derived dendritic cells promote T follicular helper cell differentiation
- PMID: 24737871
- PMCID: PMC4023883
- DOI: 10.1002/emmm.201403841
Monocyte-derived dendritic cells promote T follicular helper cell differentiation
Abstract
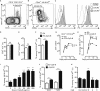
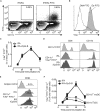
To be effective, protein priming must induce the development of a distinct lineage of CD4(+) T cells named T follicular helper (Tfh) cells, which regulate the differentiation of high-affinity memory B cells and long-lived plasma cells. In this context, we tested how adjuvantation with CpG, the Toll-like receptor 9 agonist used in clinics, contributes to antigen-specific T-cell-dependent B-cell responses in vivo. We found that addition of CpG to other vaccine adjuvant increased the differentiation of antigen-specific Tfh cells without changing the overall magnitude of the T-cell response. This phenomenon correlated with an enhancement of the germinal centre reaction, antigen-specific plasma cells and circulating antibodies. We comprehensively demonstrated that, in addition to the classical Tfh-cell differentiation mediated by conventional DC, the promoting effect due to CpG was orchestrated in vivo by antigen presentation and IL-6 secreted by monocyte-derived dendritic cells (DC) as shown in their absence. Thus, while conventional DC initiate T-cell responses, targeting monocyte-derived DC specifically enhances the Tfh programme needed to regulate high-affinity B-cell protection in vivo.
Figures



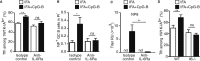

Comment in
-
Monocyte-derived dendritic cells identified as booster of T follicular helper cell differentiation.EMBO Mol Med. 2014 May;6(5):574-6. doi: 10.1002/emmm.201404015. EMBO Mol Med. 2014. PMID: 24803394 Free PMC article.
References
-
- Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–107. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. - PubMed
-
- Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O'Hagan DT, De Gregorio E, Seubert A, Wack A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29:1812–1823. - PubMed
-
- Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

