Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies
- PMID: 24738035
- PMCID: PMC3969803
- DOI: 10.7497/j.issn.2095-3941.2014.01.001
Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies
Abstract
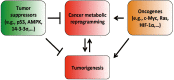
Cancer cells are well documented to rewire their metabolism and energy production networks to support and enable rapid proliferation, continuous growth, survival in harsh conditions, invasion, metastasis, and resistance to cancer treatments. Since Dr. Otto Warburg's discovery about altered cancer cell metabolism in 1930, thousands of studies have shed light on various aspects of cancer metabolism with a common goal to find new ways for effectively eliminating tumor cells by targeting their energy metabolism. This review highlights the importance of the main features of cancer metabolism, summarizes recent remarkable advances in this field, and points out the potentials to translate these scientific findings into life-saving diagnosis and therapies to help cancer patients.
Keywords: Cell cycle; energy metabolism; glutaminolysis; glycolysis; mitochondria biogenesis.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
