The design of a quantitative western blot experiment
- PMID: 24738055
- PMCID: PMC3971489
- DOI: 10.1155/2014/361590
The design of a quantitative western blot experiment
Abstract
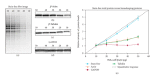
Western blotting is a technique that has been in practice for more than three decades that began as a means of detecting a protein target in a complex sample. Although there have been significant advances in both the imaging and reagent technologies to improve sensitivity, dynamic range of detection, and the applicability of multiplexed target detection, the basic technique has remained essentially unchanged. In the past, western blotting was used simply to detect a specific target protein in a complex mixture, but now journal editors and reviewers are requesting the quantitative interpretation of western blot data in terms of fold changes in protein expression between samples. The calculations are based on the differential densitometry of the associated chemiluminescent and/or fluorescent signals from the blots and this now requires a fundamental shift in the experimental methodology, acquisition, and interpretation of the data. We have recently published an updated approach to produce quantitative densitometric data from western blots (Taylor et al., 2013) and here we summarize the complete western blot workflow with a focus on sample preparation and data analysis for quantitative western blotting.
Figures

References
-
- Penque D. Two-dimensional gel electrophoresis and mass spectrometry for biomarker discovery. Proteomics—Clinical Applications. 2009;3(2):155–172. - PubMed
-
- Dubitsky A, DeCollibus D, Ortolano GA. Sensitive fluorescent detection of protein on nylon membranes. Journal of Biochemical and Biophysical Methods. 2002;51(1):47–56. - PubMed
-
- McDonald K. Increase Western blot throughput with multiplex fluorescent detection: advice and tips on how to quickly generate reliable and reproducible results. Pharma. 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

