Parahydrogen induced polarization of 1-(13)C-phospholactate-d(2) for biomedical imaging with >30,000,000-fold NMR signal enhancement in water
- PMID: 24738968
- PMCID: PMC4063326
- DOI: 10.1021/ac500952z
Parahydrogen induced polarization of 1-(13)C-phospholactate-d(2) for biomedical imaging with >30,000,000-fold NMR signal enhancement in water
Abstract
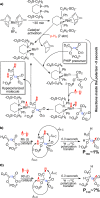
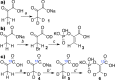
The synthetic protocol for preparation of 1-(13)C-phosphoenolpyruvate-d2, precursor for parahydrogen-induced polarization (PHIP) of 1-(13)C-phospholactate-d2, is reported. (13)C nuclear spin polarization of 1-(13)C-phospholactate-d2 was increased by >30,000,000-fold (5.75 mT) in water. The reported (13)C polarization level approaching unity (>15.6%), long lifetime of (13)C hyperpolarized 1-(13)C-phospholactate-d2 (58 ± 4 s versus 36 ± 2 s for nondeuterated form at 47.5 mT), and large production quantities (52 μmoles in 3 mL) in aqueous medium make this compound useful as a potential contrast agent for the molecular imaging of metabolism and other applications.
Figures


References
-
- Goldman M.; Johannesson H. C. R. Phys. 2005, 6, 575–581.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

