Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence
- PMID: 24739385
- PMCID: PMC4140937
- DOI: 10.1074/jbc.M113.530212
Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence
Abstract
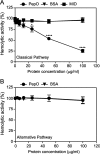
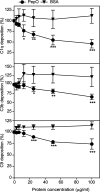
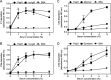
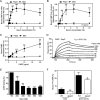
The Gram-positive species Streptococcus pneumoniae is a human pathogen causing severe local and life-threatening invasive diseases associated with high mortality rates and death. We demonstrated recently that pneumococcal endopeptidase O (PepO) is a ubiquitously expressed, multifunctional plasminogen and fibronectin-binding protein facilitating host cell invasion and evasion of innate immunity. In this study, we found that PepO interacts directly with the complement C1q protein, thereby attenuating the classical complement pathway and facilitating pneumococcal complement escape. PepO binds both free C1q and C1 complex in a dose-dependent manner based on ionic interactions. Our results indicate that recombinant PepO specifically inhibits the classical pathway of complement activation in both hemolytic and complement deposition assays. This inhibition is due to direct interaction of PepO with C1q, leading to a strong activation of the classical complement pathway, and results in consumption of complement components. In addition, PepO binds the classical complement pathway inhibitor C4BP, thereby regulating downstream complement activation. Importantly, pneumococcal surface-exposed PepO-C1q interaction mediates bacterial adherence to host epithelial cells. Taken together, PepO facilitates C1q-mediated bacterial adherence, whereas its localized release consumes complement as a result of its activation following binding of C1q, thus representing an additional mechanism of human complement escape by this versatile pathogen.
Keywords: Cell Adhesion; Complement Activation; Complement System; Host Cell Adherence; Host-Pathogen Interaction; Innate Immunity; Pneumococci; Streptococcus; c1q.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Hackel M., Lascols C., Bouchillon S., Hilton B., Morgenstern D., Purdy J. (2013) Serotype prevalence and antibiotic resistance in Streptococcus pneumoniae clinical isolates among global populations. Vaccine 31, 4881–4887 - PubMed
-
- O'Brien K. L., Wolfson L. J., Watt J. P., Henkle E., Deloria-Knoll M., McCall N., Lee E., Mulholland K., Levine O. S., Cherian T. (2009) Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374, 893–902 - PubMed
-
- Blom A. M., Hallström T., Riesbeck K. (2009) Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol. Immunol. 46, 2808–2817 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

