Risk-proportionate clinical trial monitoring: an example approach from a non-commercial trials unit
- PMID: 24739398
- PMCID: PMC4022377
- DOI: 10.1186/1745-6215-15-127
Risk-proportionate clinical trial monitoring: an example approach from a non-commercial trials unit
Abstract
Background: Some level of monitoring is usually required during a clinical trial to protect the rights and safety of trial participants and to safeguard the quality and reliability of trial results. Although there is increasing support for the use of risk-proportionate approaches to achieve these aims, the variety of methods and lack of an empirical evidence base can present challenges for clinical trial practitioners.
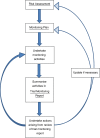
Methods: This paper describes the monitoring methods and procedures that are utilised by a non-commercial clinical trials unit which coordinates a range of clinical trials across a variety of clinical areas with different associated risks.
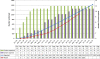
Results: Monitoring activities and approaches should be selected to be proportionate to the risks identified within a trial. A risk-proportionate approach to monitoring is described giving details of methods that may be considered by clinical trial practitioners during the development of a trial monitoring plan. An example risk assessment and corresponding monitoring plan for a low risk (type A in the Medicines and Healthcare Products Regulatory Agency (MHRA) classification system) pediatric trial is provided for illustration.
Conclusion: We present ideas for developing a monitoring plan for a clinical trial of an investigational medicinal product based on our experience. Alternative approaches may be relevant or preferable in other settings based on inherent risk.
Figures

References
-
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6(R1) 1996. [ http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Eff...]
-
- Effective and Efficient Monitoring as a Component of Quality. [ http://ctti-clinicaltrials.org/what-we-do/study-conduct/monitoring]
-
- MRC/DH/MHRA Joint Project. Risk-adapted Approaches to the Management of Clinical Trials of Investigational Medicinal Products. 2011. http://www.mhra.gov.uk/home/groups/l-ctu/documents/websiteresources/con1....
-
- Food and Drug Administration. Guidance for Industry Oversight of Clinical Investigations — A Risk-Based Approach to Monitoring. 2013. [ http://www.fda.gov/downloads/Drugs/…/Guidances/UCM269919.pdf Accessed 19/12/2013]
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

