Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread
- PMID: 24739967
- PMCID: PMC4151619
- DOI: 10.1038/nature13168
Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread
Abstract
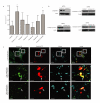
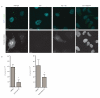
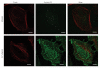
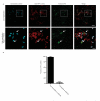
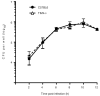
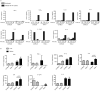
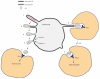
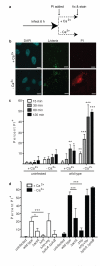
Efferocytosis, the process by which dying or dead cells are removed by phagocytosis, has an important role in development, tissue homeostasis and innate immunity. Efferocytosis is mediated, in part, by receptors that bind to exofacial phosphatidylserine (PS) on cells or cellular debris after loss of plasma membrane asymmetry. Here we show that a bacterial pathogen, Listeria monocytogenes, can exploit efferocytosis to promote cell-to-cell spread during infection. These bacteria can escape the phagosome in host cells by using the pore-forming toxin listeriolysin O (LLO) and two phospholipase C enzymes. Expression of the cell surface protein ActA allows L. monocytogenes to activate host actin regulatory factors and undergo actin-based motility in the cytosol, eventually leading to formation of actin-rich protrusions at the cell surface. Here we show that protrusion formation is associated with plasma membrane damage due to LLO's pore-forming activity. LLO also promotes the release of bacteria-containing protrusions from the host cell, generating membrane-derived vesicles with exofacial PS. The PS-binding receptor TIM-4 (encoded by the Timd4 gene) contributes to efficient cell-to-cell spread by L. monocytogenes in macrophages in vitro and growth of these bacteria is impaired in Timd4(-/-) mice. Thus, L. monocytogenes promotes its dissemination in a host by exploiting efferocytosis. Our results indicate that PS-targeted therapeutics may be useful in the fight against infections by L. monocytogenes and other bacteria that use similar strategies of cell-to-cell spread during infection.
Figures



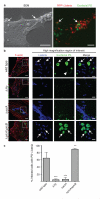

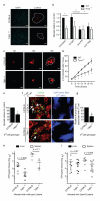
Comment in
-
Bacterial pathogenesis: Pirate bacteria hijack efferocytosis.Nat Rev Microbiol. 2014 Jun;12(6):396-7. doi: 10.1038/nrmicro3272. Epub 2014 Apr 28. Nat Rev Microbiol. 2014. PMID: 24769867 No abstract available.
-
Listeria exploits damage and death to spread bad news.Trends Microbiol. 2014 Jul;22(7):370-1. doi: 10.1016/j.tim.2014.06.001. Epub 2014 Jun 13. Trends Microbiol. 2014. PMID: 24934861 Free PMC article.
References
-
- Mostowy S, Cossart P. Virulence factors that modulate the cell biology of Listeria infection and the host response. Adv Immunol. 2012;113:19–32. - PubMed
-
- Alberti-Segui C, Goeden KR, Higgins DE. Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell Microbiol. 2007;9:179–195. - PubMed
Methods References
-
- Bishop DK, Hinrichs DJ. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. - PubMed

