A potent combination microbicide that targets SHIV-RT, HSV-2 and HPV
- PMID: 24740100
- PMCID: PMC3989196
- DOI: 10.1371/journal.pone.0094547
A potent combination microbicide that targets SHIV-RT, HSV-2 and HPV
Abstract
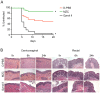
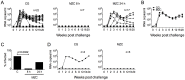
Prevalent infection with human herpes simplex 2 (HSV-2) or human papillomavirus (HPV) is associated with increased human immunodeficiency virus (HIV) acquisition. Microbicides that target HIV as well as these sexually transmitted infections (STIs) may more effectively limit HIV incidence. Previously, we showed that a microbicide gel (MZC) containing MIV-150, zinc acetate (ZA) and carrageenan (CG) protected macaques against simian-human immunodeficiency virus (SHIV-RT) infection and that a ZC gel protected mice against HSV-2 infection. Here we evaluated a modified MZC gel (containing different buffers, co-solvents, and preservatives suitable for clinical testing) against both vaginal and rectal challenge of animals with SHIV-RT, HSV-2 or HPV. MZC was stable and safe in vitro (cell viability and monolayer integrity) and in vivo (histology). MZC protected macaques against vaginal (p<0.0001) SHIV-RT infection when applied up to 8 hours (h) prior to challenge. When used close to the time of challenge, MZC prevented rectal SHIV-RT infection of macaques similar to the CG control. MZC significantly reduced vaginal (p<0.0001) and anorectal (p = 0.0187) infection of mice when 10(6) pfu HSV-2 were applied immediately after vaginal challenge and also when 5×10(3) pfu were applied between 8 h before and 4 h after vaginal challenge (p<0.0248). Protection of mice against 8×10(6) HPV16 pseudovirus particles (HPV16 PsV) was significant for MZC applied up to 24 h before and 2 h after vaginal challenge (p<0.0001) and also if applied 2 h before or after anorectal challenge (p<0.0006). MZC provides a durable window of protection against vaginal infection with these three viruses and, against HSV-2 and HPV making it an excellent candidate microbicide for clinical use.
Conflict of interest statement
Figures
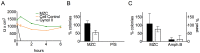
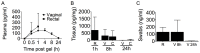


References
-
- Corey L (2007) Herpes simplex virus type 2 and HIV-1: the dialogue between the 2 organisms continues. J Infect Dis 195: 1242–1244. - PubMed
-
- Jin XW, Lipold L, Sikon A, Rome E (2013) Human papillomavirus vaccine: safe, effective, underused. Cleve Clin J Med 80: 49–60. - PubMed
-
- Nel AM, Coplan P, Smythe SC, McCord K, Mitchnick M, et al. (2010) Pharmacokinetic assessment of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS Res Hum Retroviruses 26: 1181–1190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

