Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer
- PMID: 24740322
- PMCID: PMC4058390
- DOI: 10.1158/1078-0432.CCR-13-3296
Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer
Erratum in
-
Correction: Niclosamide Inhibits Androgen Receptor Variants Expression and Overcomes Enzalutamide Resistance in Castration-Resistant Prostate Cancer.Clin Cancer Res. 2017 Jan 1;23(1):323. doi: 10.1158/1078-0432.CCR-16-2585. Clin Cancer Res. 2017. PMID: 28049163 No abstract available.
Abstract
Purpose: Enzalutamide, a second-generation antiandrogen, was recently approved for the treatment of castration-resistant prostate cancer (CRPC) in patients who no longer respond to docetaxel. Despite these advances that provide temporary respite, resistance to enzalutamide occurs frequently. Androgen receptor (AR) splice variants such as AR-V7 have recently been shown to drive castration-resistant growth and resistance to enzalutamide. This study was designed to identify inhibitors of AR variants and test its ability to overcome resistance to enzalutamide.
Experimental design: The drug screening was conducted using luciferase activity assay to determine the activity of AR-V7 after treatment with the compounds in the Prestwick Chemical Library, which contains about 1,120 FDA-approved drugs. The effects of the identified inhibitors on AR-V7 activity and enzalutamide sensitivity were characterized in CRPC and enzalutamide-resistant prostate cancer cells in vitro and in vivo.
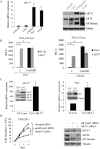
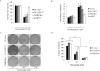
Results: Niclosamide, an FDA-approved antihelminthic drug, was identified as a potent AR-V7 inhibitor in prostate cancer cells. Niclosamide significantly downregulated AR-V7 protein expression by protein degradation through a proteasome-dependent pathway. Niclosamide also inhibited AR-V7 transcription activity and reduced the recruitment of AR-V7 to the PSA promoter. Niclosamide inhibited prostate cancer cell growth in vitro and tumor growth in vivo. Furthermore, the combination of niclosamide and enzalutamide resulted in significant inhibition of enzalutamide-resistant tumor growth, suggesting that niclosamide enhances enzalutamide therapy and overcomes enzalutamide resistance in CRPC cells.
Conclusions: Niclosamide was identified as a novel inhibitor of AR variants. Our findings offer preclinical validation of niclosamide as a promising inhibitor of AR variants to treat, either alone or in combination with current antiandrogen therapies, patients with advanced prostate cancer, especially those resistant to enzalutamide.
©2014 American Association for Cancer Research.
Figures




Comment in
-
Prostate cancer: Niclosamide jumps the hurdle of enzalutamide resistance.Nat Rev Urol. 2014 Aug;11(8):424. doi: 10.1038/nrurol.2014.160. Epub 2014 Jul 1. Nat Rev Urol. 2014. PMID: 24980198 No abstract available.
References
-
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. The New England journal of medicine. 2012;367:1187–97. - PubMed
-
- Kim W, Ryan CJ. Androgen receptor directed therapies in castration-resistant metastatic prostate cancer. Curr Treat Options Oncol. 2012;13:189–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

