Protection from intestinal inflammation by bacterial exopolysaccharides
- PMID: 24740503
- PMCID: PMC4018721
- DOI: 10.4049/jimmunol.1303369
Protection from intestinal inflammation by bacterial exopolysaccharides
Abstract
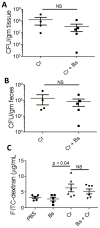
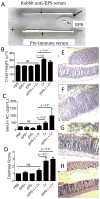
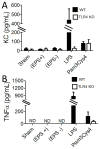
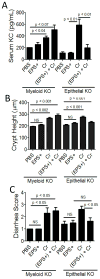
Host inflammatory responses against pathogenic organisms can be abrogated by commensals; however, the molecular mechanisms by which pathogenesis is prevented are still poorly understood. Previous studies demonstrated that administration of a single dose of Bacillus subtilis prevented disease and inflammation by the enteric mouse pathogen Citrobacter rodentium, which causes disease similar to the human pathogen enteropathogenic Escherichia coli. No protection was observed when an exopolysaccharide (EPS)-deficient mutant of B. subtilis was used, suggesting that EPS are the protective factor. In this study, we isolated and characterized EPS and showed that they also prevent C. rodentium-associated intestinal disease after a single injection. Protection is TLR4 dependent because EPS-treated TLR4 knockout mice developed disease. Furthermore, protection could be conveyed to wild-type mice by adoptive transfer of macrophage-rich peritoneal cells from EPS-treated mice. We found that EPS specifically bind peritoneal macrophages, and because mice lacking MyD88 signaling in myeloid cells were not protected by EPS, we conclude that bacterial EPS prevent colitis in a TLR4-dependent manner that requires myeloid cells. These studies provide a simple means of preventing intestinal inflammation caused by enteric pathogens.
Conflict of interest statement
Figures
References
-
- Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T, Yoshimura A, Kanai T. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722. - PubMed
-
- Gais P, Reim D, Jusek G, Rossmann-Bloeck T, Weighardt H, Pfeffer K, Altmayr F, Janssen KP, Holzmann B. Cutting edge: Divergent cell-specific functions of MyD88 for inflammatory responses and organ injury in septic peritonitis. J Immunol. 2012;188:5833–5837. - PubMed
-
- D’Arienzo R, Maurano F, Mazzarella G, Luongo D, Stefanile R, Ricca E, Rossi M. Bacillus subtilis spores reduce susceptibility to Citrobacter rodentium-mediated enteropathy in a mouse model. Res Microbiol. 2006;157:891–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

