Release of cystic fibrosis airway inflammatory markers from Pseudomonas aeruginosa-stimulated human neutrophils involves NADPH oxidase-dependent extracellular DNA trap formation
- PMID: 24740504
- PMCID: PMC4032287
- DOI: 10.4049/jimmunol.1301589
Release of cystic fibrosis airway inflammatory markers from Pseudomonas aeruginosa-stimulated human neutrophils involves NADPH oxidase-dependent extracellular DNA trap formation
Abstract
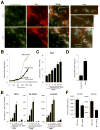
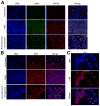
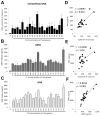
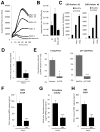
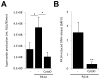
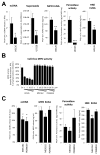
Cystic fibrosis (CF) airways are characterized by bacterial infections, excess mucus production, and robust neutrophil recruitment. The main CF airway pathogen is Pseudomonas aeruginosa. Neutrophils are not capable of clearing the infection. Neutrophil primary granule components, myeloperoxidase (MPO) and human neutrophil elastase (HNE), are inflammatory markers in CF airways, and their increased levels are associated with poor lung function. Identifying the mechanism of MPO and HNE release from neutrophils is of high clinical relevance for CF. In this article, we show that human neutrophils release large amounts of neutrophil extracellular traps (NETs) in the presence of P. aeruginosa. Bacteria are entangled in NETs and colocalize with extracellular DNA. MPO, HNE, and citrullinated histone H4 are all associated with DNA in Pseudomonas-triggered NETs. Both laboratory standard strains and CF isolates of P. aeruginosa induce DNA, MPO, and HNE release from human neutrophils. The increase in peroxidase activity of neutrophil supernatants after Pseudomonas exposure indicates that enzymatically active MPO is released. P. aeruginosa induces a robust respiratory burst in neutrophils that is required for extracellular DNA release. Inhibition of the cytoskeleton prevents Pseudomonas-initiated superoxide production and DNA release. NADPH oxidase inhibition suppresses Pseudomonas-induced release of active MPO and HNE. Blocking MEK/ERK signaling results in only minimal inhibition of DNA release induced by Pseudomonas. Our data describe in vitro details of DNA, MPO, and HNE release from neutrophils activated by P. aeruginosa. We propose that Pseudomonas-induced NET formation is an important mechanism contributing to inflammatory conditions characteristic of CF airways.
Figures
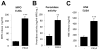
Similar articles
-
NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes.Immunol Lett. 2014 Aug;160(2):186-94. doi: 10.1016/j.imlet.2014.03.003. Epub 2014 Mar 23. Immunol Lett. 2014. PMID: 24670966
-
Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR.PLoS One. 2011;6(9):e23637. doi: 10.1371/journal.pone.0023637. Epub 2011 Sep 1. PLoS One. 2011. PMID: 21909403 Free PMC article.
-
Systemic levels of anti-PAD4 autoantibodies correlate with airway obstruction in cystic fibrosis.J Cyst Fibros. 2019 Sep;18(5):636-645. doi: 10.1016/j.jcf.2018.12.010. Epub 2019 Jan 10. J Cyst Fibros. 2019. PMID: 30638826 Free PMC article.
-
Harnessing Neutrophil Survival Mechanisms during Chronic Infection by Pseudomonas aeruginosa: Novel Therapeutic Targets to Dampen Inflammation in Cystic Fibrosis.Front Cell Infect Microbiol. 2017 Jun 30;7:243. doi: 10.3389/fcimb.2017.00243. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713772 Free PMC article. Review.
-
Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation.Genes (Basel). 2019 Feb 26;10(3):183. doi: 10.3390/genes10030183. Genes (Basel). 2019. PMID: 30813645 Free PMC article. Review.
Cited by
-
NETosis in the pathogenesis of acute lung injury following cutaneous chemical burns.JCI Insight. 2021 May 24;6(10):e147564. doi: 10.1172/jci.insight.147564. JCI Insight. 2021. PMID: 34027893 Free PMC article.
-
Cystic fibrosis autoantibody signatures associate with Staphylococcus aureus lung infection or cystic fibrosis-related diabetes.Front Immunol. 2023 Sep 11;14:1151422. doi: 10.3389/fimmu.2023.1151422. eCollection 2023. Front Immunol. 2023. PMID: 37767091 Free PMC article.
-
Swimming Motility Mediates the Formation of Neutrophil Extracellular Traps Induced by Flagellated Pseudomonas aeruginosa.PLoS Pathog. 2016 Nov 17;12(11):e1005987. doi: 10.1371/journal.ppat.1005987. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27855208 Free PMC article.
-
NETs and CF Lung Disease: Current Status and Future Prospects.Antibiotics (Basel). 2015 Jan 15;4(1):62-75. doi: 10.3390/antibiotics4010062. Antibiotics (Basel). 2015. PMID: 27025615 Free PMC article. Review.
-
Antibodies Against Pseudomonas aeruginosa Alkaline Protease Directly Enhance Disruption of Neutrophil Extracellular Traps Mediated by This Enzyme.Front Immunol. 2021 Mar 31;12:654649. doi: 10.3389/fimmu.2021.654649. eCollection 2021. Front Immunol. 2021. PMID: 33868297 Free PMC article.
References
-
- Kim Chiaw P, Eckford PD, Bear CE. Insights into the mechanisms underlying CFTR channel activity, the molecular basis for cystic fibrosis and strategies for therapy. Essays in biochemistry. 2011;50:233–248. - PubMed
-
- Ciofu O, Hansen CR, Hoiby N. Respiratory bacterial infections in cystic fibrosis. Current opinion in pulmonary medicine. 2013;19:251–258. - PubMed
-
- West SE, Zeng L, Lee BL, Kosorok MR, Laxova A, Rock MJ, Splaingard MJ, Farrell PM. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA : the journal of the American Medical Association. 2002;287:2958–2967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

