Tissue-resident exhausted effector memory CD8+ T cells accumulate in the retina during chronic experimental autoimmune uveoretinitis
- PMID: 24740509
- PMCID: PMC4009498
- DOI: 10.4049/jimmunol.1301390
Tissue-resident exhausted effector memory CD8+ T cells accumulate in the retina during chronic experimental autoimmune uveoretinitis
Abstract
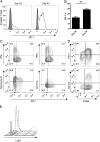
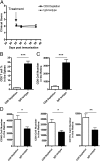
Experimental autoimmune uveoretinitis is a model for noninfectious posterior segment intraocular inflammation in humans. Although this disease is CD4(+) T cell dependent, in the persistent phase of disease CD8(+) T cells accumulate. We show that these are effector memory CD8(+) T cells that differ from their splenic counterparts with respect to surface expression of CD69, CD103, and Ly6C. These retinal effector memory CD8(+) T cells have limited cytotoxic effector function, are impaired in their ability to proliferate in response to Ag-specific stimulation, and upregulate programmed death 1 receptor. Treatment with fingolimod (FTY720) during the late phase of disease revealed that retinal CD8(+) T cells were tissue resident. Despite signs of exhaustion, these cells were functional, as their depletion resulted in an expansion of retinal CD4(+) T cells and CD11b(+) macrophages. These results demonstrate that, during chronic autoimmune inflammation, exhausted CD8(+) T cells become established in the local tissue. They are phenotypically distinct from peripheral CD8(+) T cells and provide local signals within the tissue by expression of inhibitory receptors such as programmed death 1 that limit persistent inflammation.
Figures





References
-
- Imrie F. R., Dick A. D. 2007. Biologics in the treatment of uveitis. Curr. Opin. Ophthalmol. 18: 481–486. - PubMed
-
- Avichezer D., Silver P. B., Chan C. C., Wiggert B., Caspi R. R. 2000. Identification of a new epitope of human IRBP that induces autoimmune uveoretinitis in mice of the H-2b haplotype. Invest. Ophthalmol. Vis. Sci. 41: 127–131. - PubMed
-
- Kerr E. C., Raveney B. J., Copland D. A., Dick A. D., Nicholson L. B. 2008. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J. Autoimmun. 31: 354–361. - PubMed
-
- Chen, M., D. A. Copland, J. Zhao, J. Liu, J. V. Forrester, A. D. Dick, and H. Xu. 2012. Persistent inflammation subverts thrombospondin-1-induced regulation of retinal angiogenesis and is driven by CCR2 ligation. Am. J. Pathol. 180: 235-245. - PubMed
-
- Forrester J. V., Huitinga I., Lumsden L., Dijkstra C. D. 1998. Marrow-derived activated macrophages are required during the effector phase of experimental autoimmune uveoretinitis in rats. Curr. Eye Res. 17: 426–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

