Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis
- PMID: 24740535
- PMCID: PMC4422352
- DOI: 10.1152/ajpcell.00321.2013
Cellular mechanisms of tissue fibrosis. 7. New insights into the cellular mechanisms of pulmonary fibrosis
Abstract
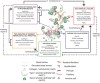
Idiopathic pulmonary fibrosis (IPF) is a devastating disease characterized by severe and progressive scar formation in the gas-exchange regions of the lung. Despite years of research, therapeutic treatments remain elusive and there is a pressing need for deeper mechanistic insights into the pathogenesis of the disease. In this article, we review our current knowledge of the triggers and/or perpetuators of pulmonary fibrosis with special emphasis on the alveolar epithelium and the underlying mesenchyme. In doing so, we raise a number of questions highlighting critical voids and limitations in our current understanding and study of this disease.
Keywords: epithelium; lung fibrosis; myofibroblast.
Copyright © 2014 the American Physiological Society.
Figures

References
-
- Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, Hamosh A, Greider CW. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci USA 102: 15960–15964, 2005. - PMC - PubMed
-
- Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 356: 1317–1326, 2007. - PubMed
-
- Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, Sato A, Kudoh S. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 171: 1040–1047, 2005. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

