Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: results from a randomized controlled trial
- PMID: 24740596
- PMCID: PMC4277330
- DOI: 10.1002/ijc.28897
Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: results from a randomized controlled trial
Abstract
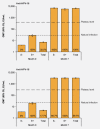
This phase II/III, double-blind, randomized trial assessed the efficacy, immunogenicity and safety of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in young Chinese women (ClinicalTrials.gov registration NCT00779766). Women aged 18-25 years from Jiangsu province were randomized (1:1) to receive HPV vaccine (n = 3,026) or Al(OH)3 control (n = 3,025) at months 0, 1 and 6. The primary objective was vaccine efficacy (VE) against HPV-16/18 associated 6-month persistent infection (PI) and/or cervical intraepithelial neoplasia (CIN) 1+. Secondary objectives were VE against virological and clinical endpoints associated with HPV-16/18 and with high-risk HPV types, immunogenicity and safety. Mean follow-up for the according-to-protocol cohort for efficacy (ATP-E) was ∼15 months after the third dose. In the ATP-E (vaccine = 2,889; control = 2,894), for initially HPV DNA negative and seronegative subjects, HPV-16/18 related VE (95% CI) was 94.2% (62.7, 99.9) against 6-month PI and/or CIN1+ and 93.8% (60.2, 99.9) against cytological abnormalities. VE against HPV-16/18 associated CIN1+ and CIN2+ was 100% (-50.4, 100) and 100% (-140.2, 100), respectively (no cases in the vaccine group and 4 CIN1+ and 3 CIN2+ cases in the control group). At Month 7, at least 99.7% of initially seronegative vaccine recipients had seroconverted for HPV-16/18; geometric mean antibody titres (95% CI) were 6,996 (6,212 to 7,880) EU/mL for anti-HPV-16 and 3,309 (2,942 to 3,723) EU/mL for anti-HPV-18. Safety outcomes between groups were generally similar. The HPV-16/18 AS04-adjuvanted vaccine is effective, immunogenic and has a clinically acceptable safety profile in young Chinese women. Prophylactic HPV vaccination has the potential to substantially reduce the burden of cervical cancer in China.
Keywords: China; efficacy; human papillomavirus vaccine; immunogenicity; safety.
© 2014 The Authors. Published by Wiley Periodicals, Inc. on behalf of UICC.
Figures
References
-
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. Available at http://globocan.iarc.fr. Accessed on: 7 August 2013.
-
- Bao YP, Li N, Smith JS, et al. Human papillomavirus type-distribution in the cervix of Chinese women: a meta-analysis. Int J STD AIDS. 2008;19:106–11. - PubMed
-
- Chen W, Zhang X, Molijn A, et al. Human papillomavirus type-distribution in cervical cancer in China: the importance of HPV 16 and 18. Cancer Causes Control. 2009;20:1705–13. - PubMed
-
- Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

