Adenine nucleotide metabolism and a role for AMP in modulating flagellar waveforms in mouse sperm
- PMID: 24740601
- PMCID: PMC4094002
- DOI: 10.1095/biolreprod.113.114447
Adenine nucleotide metabolism and a role for AMP in modulating flagellar waveforms in mouse sperm
Abstract
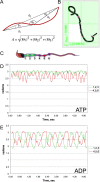
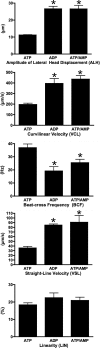
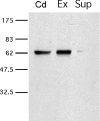
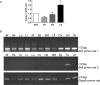
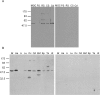
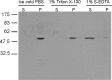
While most ATP, the main energy source driving sperm motility, is derived from glycolysis and oxidative phosphorylation, the metabolic demands of the cell require the efficient use of power stored in high-energy phosphate bonds. In times of high energy consumption, adenylate kinase (AK) scavenges one ATP molecule by transphosphorylation of two molecules of ADP, simultaneously yielding one molecule of AMP as a by-product. Either ATP or ADP supported motility of detergent-modeled cauda epididymal mouse sperm, indicating that flagellar AKs are functional. However, the ensuing flagellar waveforms fueled by ATP or ADP were qualitatively different. Motility driven by ATP was rapid but restricted to the distal region of the sperm tail, whereas ADP produced slower and more fluid waves that propagated down the full flagellum. Characterization of wave patterns by tracing and superimposing the images of the flagella, quantifying the differences using digital image analysis, and computer-assisted sperm analysis revealed differences in the amplitude, periodicity, and propagation of the waves between detergent-modeled sperm treated with either ATP or ADP. Surprisingly, addition of AMP to the incubation medium containing ATP recapitulated the pattern of sperm motility seen with ADP alone. In addition to AK1 and AK2, which we previously demonstrated are present in outer dense fibers and mitochondrial sheath of the mouse sperm tail, we show that another AK, AK8, is present in a third flagellar compartment, the axoneme. These results extend the known regulators of sperm motility to include AMP, which may be operating through an AMP-activated protein kinase.
Keywords: ADP; AK8; AMP; ATP; adenine nucleotides; adenylate kinase; motility; sperm.
© 2014 by the Society for the Study of Reproduction, Inc.
Figures
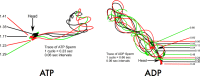


Similar articles
-
Adenylate kinase phosphate energy shuttle underlies energetic communication in flagellar axonemes.Sci China Life Sci. 2024 Aug;67(8):1697-1714. doi: 10.1007/s11427-023-2539-1. Epub 2024 May 16. Sci China Life Sci. 2024. PMID: 38761355
-
Adenylate kinases 1 and 2 are part of the accessory structures in the mouse sperm flagellum.Biol Reprod. 2006 Oct;75(4):492-500. doi: 10.1095/biolreprod.106.053512. Epub 2006 Jun 21. Biol Reprod. 2006. PMID: 16790685
-
Adenylate kinase 1 deficiency disrupts mouse sperm motility under conditions of energy stress†.Biol Reprod. 2020 Oct 29;103(5):1121-1131. doi: 10.1093/biolre/ioaa134. Biol Reprod. 2020. PMID: 32744313
-
Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use?Asian J Androl. 2015 Mar-Apr;17(2):230-5. doi: 10.4103/1008-682X.135123. Asian J Androl. 2015. PMID: 25475660 Free PMC article. Review.
-
Functional anatomy of the mammalian sperm flagellum.Cytoskeleton (Hoboken). 2016 Nov;73(11):652-669. doi: 10.1002/cm.21338. Epub 2016 Oct 21. Cytoskeleton (Hoboken). 2016. PMID: 27712041 Review.
Cited by
-
Regulatory processes that control haploid expression of salmon sperm mRNAs.BMC Res Notes. 2018 Sep 3;11(1):639. doi: 10.1186/s13104-018-3749-z. BMC Res Notes. 2018. PMID: 30176937 Free PMC article.
-
Caput Ligation Renders Immature Mouse Sperm Motile and Capable to Undergo cAMP-Dependent Phosphorylation.Int J Mol Sci. 2021 Sep 23;22(19):10241. doi: 10.3390/ijms221910241. Int J Mol Sci. 2021. PMID: 34638585 Free PMC article.
-
Changes in the Adenylate Kinase Activity are Proportional to the ADP/ATP Ratio Upon Resorption and Regeneration of Chlamydomonas reinhardtii Flagella.Cell Biochem Biophys. 2025 Jul 18. doi: 10.1007/s12013-025-01825-z. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 40679766
-
Label-free proteomic comparison reveals ciliary and nonciliary phenotypes of IFT-A mutants.Mol Biol Cell. 2024 Mar 1;35(3):ar39. doi: 10.1091/mbc.E23-03-0084. Epub 2024 Jan 3. Mol Biol Cell. 2024. PMID: 38170584 Free PMC article.
-
Genetic causes of male infertility: snapshot on morphological abnormalities of the sperm flagellum.Basic Clin Androl. 2019 Mar 4;29:2. doi: 10.1186/s12610-019-0083-9. eCollection 2019. Basic Clin Androl. 2019. PMID: 30867909 Free PMC article.
References
-
- Krisfalusi M, Miki K, Magyar PL, O'Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod 2006; 75 (2): 270–278. - PubMed
-
- Cao W, Haig-Ladewig L, Gerton GL, Moss SB. Adenylate kinases 1 and 2 are part of the accessory structures in the mouse sperm flagellum. Biol Reprod 2006; 75 (4): 492–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

