HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial
- PMID: 24740633
- PMCID: PMC4176446
- DOI: 10.1093/infdis/jiu233
HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial
Abstract
Background: The iPrEx study demonstrated that combination oral emtricitabine and tenofovir disoproxil fumarate (FTC/TDF) as preexposure prophylaxis (PrEP) protects against HIV acquisition in men who have sex with men and transgender women. Selection for drug resistance could offset PrEP benefits.
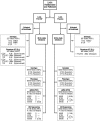
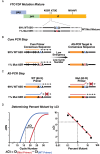
Methods: Phenotypic and genotypic clinical resistance assays characterized major drug resistant mutations. Minor variants with FTC/TDF mutations K65R, K70E, M184V/I were measured using 454 deep sequencing and a novel allele-specific polymerase chain reaction (AS-PCR) diagnostic tolerant to sequence heterogeneity.
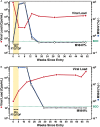
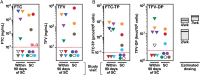
Results: Control of primer-binding site heterogeneity resulted in improved accuracy of minor variant measurements by AS-PCR. Of the 48 on-study infections randomized to FTC/TDF, none showed FTC/TDF mutations by clinical assays despite detectable drug levels in 8 participants. Two randomized to FTC/TDF had minor variant M184I detected at 0.53% by AS-PCR or 0.75% by deep sequencing, only 1 of which had low but detectable drug levels. Among those with acute infection at randomization to FTC/TDF, M184V or I mutations that were predominant at seroconversion waned to background levels within 24 weeks after discontinuing drug.
Conclusions: Drug resistance was rare in iPrEx on-study FTC/TDF-randomized seroconverters, and only as low-frequency minor variants. FTC resistance among those initiating PrEP with acute infection waned rapidly after drug discontinuation. Clinical Trials Registration.NCT00458393.
Keywords: 454 deep sequencing; AS-PCR; FTC/TDF; HIV-1; PrEP; drug resistance; minor variant; preexposure prophylaxis.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. - PubMed
-
- Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–90. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

