Overexpression of TRB3 in muscle alters muscle fiber type and improves exercise capacity in mice
- PMID: 24740654
- PMCID: PMC4159733
- DOI: 10.1152/ajpregu.00027.2014
Overexpression of TRB3 in muscle alters muscle fiber type and improves exercise capacity in mice
Abstract
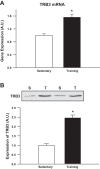
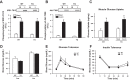
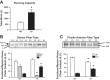
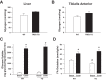
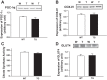
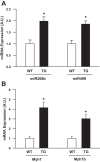
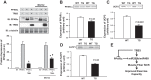
Increasing evidence suggests that TRB3, a mammalian homolog of Drosophila tribbles, plays an important role in cell growth, differentiation, and metabolism. In the liver, TRB3 binds and inhibits Akt activity, whereas in adipocytes, TRB3 upregulates fatty acid oxidation. In cultured muscle cells, TRB3 has been identified as a potential regulator of insulin signaling. However, little is known about the function and regulation of TRB3 in skeletal muscle in vivo. In the current study, we found that 4 wk of voluntary wheel running (6.6 ± 0.4 km/day) increased TRB3 mRNA by 1.6-fold and protein by 2.5-fold in the triceps muscle. Consistent with this finding, muscle-specific transgenic mice that overexpress TRB3 (TG) had a pronounced increase in exercise capacity compared with wild-type (WT) littermates (TG: 1,535 ± 283; WT: 644 ± 67 joules). The increase in exercise capacity in TRB3 TG mice was not associated with changes in glucose uptake or glycogen levels; however, these mice displayed a dramatic shift toward a more oxidative/fatigue-resistant (type I/IIA) muscle fiber type, including threefold more type I fibers in soleus muscles. Skeletal muscle from TRB3 TG mice had significantly decreased PPARα expression, twofold higher levels of miR208b and miR499, and corresponding increases in the myosin heavy chain isoforms Myh7 and Myb7b, which encode these microRNAs. These findings suggest that TRB3 regulates muscle fiber type via a peroxisome proliferator-activated receptor-α (PPAR-α)-regulated miR499/miR208b pathway, revealing a novel function for TRB3 in the regulation of skeletal muscle fiber type and exercise capacity.
Keywords: PPAR-α; TRB3; exercise capacity; microRNAs; muscle.
Copyright © 2014 the American Physiological Society.
Figures

References
-
- Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, Shulman GI. In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J Biol Chem 276: 20240–20244, 2001 - PubMed
-
- Conley DL, Krahenbuhl GS. Running economy and distance running performance of highly trained athletes. Med Sci Sports Exerc 12: 357–360, 1980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

