Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis
- PMID: 24740812
- PMCID: PMC4041169
- DOI: 10.1182/blood-2014-02-554634
Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis
Abstract
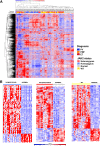
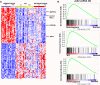
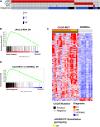
Genomic studies have identified somatic alterations in the majority of myeloproliferative neoplasms (MPN) patients, including JAK2 mutations in the majority of MPN patients and CALR mutations in JAK2-negative MPN patients. However, the role of JAK-STAT pathway activation in different MPNs, and in patients without JAK2 mutations, has not been definitively delineated. We used expression profiling, single nucleotide polymorphism arrays, and mutational profiling to investigate a well-characterized cohort of MPN patients. MPN patients with homozygous JAK2V617F mutations were characterized by a distinctive transcriptional profile. Notably, a transcriptional signature consistent with activated JAK2 signaling is seen in all MPN patients regardless of clinical phenotype or mutational status. In addition, the activated JAK2 signature was present in patients with somatic CALR mutations. Conversely, we identified a gene expression signature of CALR mutations; this signature was significantly enriched in JAK2-mutant MPN patients consistent with a shared mechanism of transformation by JAK2 and CALR mutations. We also identified a transcriptional signature of TET2 mutations in MPN patent samples. Our data indicate that MPN patients, regardless of diagnosis or JAK2 mutational status, are characterized by a distinct gene expression signature with upregulation of JAK-STAT target genes, demonstrating the central importance of the JAK-STAT pathway in MPN pathogenesis.
© 2014 by The American Society of Hematology.
Figures

References
-
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, et al. Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. - PubMed
-
- Zhao ZJ, Vainchenker W, Krantz SB, Casadevall N, Constantinescu SN. Role of tyrosine kinases and phosphatases in polycythemia vera. Semin Hematol. 2005;42(4):221–229. - PubMed
-
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

