Teriflunomide and its mechanism of action in multiple sclerosis
- PMID: 24740824
- PMCID: PMC4003395
- DOI: 10.1007/s40265-014-0212-x
Teriflunomide and its mechanism of action in multiple sclerosis
Abstract
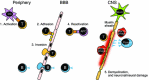
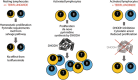
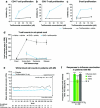
Treatment of multiple sclerosis (MS) is challenging: disease-modifying treatments (DMTs) must both limit unwanted immune responses associated with disease initiation and propagation (as T and B lymphocytes are critical cellular mediators in the pathophysiology of relapsing MS), and also have minimal adverse impact on normal protective immune responses. In this review, we summarize key preclinical and clinical data relating to the proposed mechanism of action of the recently approved DMT teriflunomide in MS. Teriflunomide selectively and reversibly inhibits dihydro-orotate dehydrogenase, a key mitochondrial enzyme in the de novo pyrimidine synthesis pathway, leading to a reduction in proliferation of activated T and B lymphocytes without causing cell death. Results from animal experiments modelling the immune activation implicated in MS demonstrate reductions in disease symptoms with teriflunomide treatment, accompanied by reduced central nervous system lymphocyte infiltration, reduced axonal loss, and preserved neurological functioning. In agreement with the results obtained in these model systems, phase 3 clinical trials of teriflunomide in patients with MS have consistently shown that teriflunomide provides a therapeutic benefit, and importantly, does not cause clinical immune suppression. Taken together, these data demonstrate how teriflunomide acts as a selective immune therapy for patients with MS.
Figures
References
-
- Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. - PubMed
-
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. - PubMed
-
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. - PubMed
-
- McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

