Dissecting the mechanisms underlying the accumulation of mitochondrial DNA deletions in human skeletal muscle
- PMID: 24740879
- PMCID: PMC4119413
- DOI: 10.1093/hmg/ddu176
Dissecting the mechanisms underlying the accumulation of mitochondrial DNA deletions in human skeletal muscle
Abstract
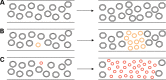
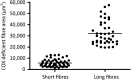
Large-scale mitochondrial DNA (mtDNA) deletions are an important cause of mitochondrial disease, while somatic mtDNA deletions cause focal respiratory chain deficiency associated with ageing and neurodegenerative disorders. As mtDNA deletions only cause cellular pathology at high levels of mtDNA heteroplasmy, an mtDNA deletion must accumulate to levels which can result in biochemical dysfunction-a process known as clonal expansion. A number of hypotheses have been proposed for clonal expansion of mtDNA deletions, including a replicative advantage for deleted mitochondrial genomes inferred by their smaller size--implying that the largest mtDNA deletions would also display a replicative advantage over smaller mtDNA deletions. We proposed that in muscle fibres from patients with mtDNA maintenance disorders, which lead to the accumulation of multiple mtDNA deletions, we would observe the largest mtDNA deletions spreading the furthest longitudinally through individual muscle fibres by means of a greater rate of clonal expansion. We characterized mtDNA deletions in patients with mtDNA maintenance disorders from a range of 'large' and 'small' cytochrome c oxidase (COX)-deficient regions in skeletal muscle fibres. We measured the size of clonally expanded deletions in 62 small and 60 large individual COX-deficient f regions. No significant difference was observed in individual patients or in the total dataset (small fibre regions mean 6.59 kb--large fibre regions mean 6.51 kb). Thus no difference existed in the rate of clonal expansion throughout muscle fibres between mtDNA deletions of different sizes; smaller mitochondrial genomes therefore do not appear to have an inherent replicative advantage in human muscle.
© The Author 2014. Published by Oxford University Press.
Figures



References
-
- Schaefer A.M., McFarland R., Blakely E.L., He L., Whittaker R.G., Taylor R.W., Chinnery P.F., Turnbull D.M. Prevalence of mitochondrial DNA disease in adults. Ann. Neurol. 2008;63:35–39. - PubMed
-
- Reeve A.K., Krishnan K.J., Turnbull D. In: Mitochondria and Oxidative Stress in Neurodegenerative Disorders. Gibson G.E., Ratan R.R., Beal M.F., editors. Vol. 1147. Oxford: Blackwell Publishing; 2008. pp. 21–29.
-
- Larsson N. Somatic mitochondrial DNA mutations in mammalian aging. Annu. Rev. Biochem. 2010;79:683–706. - PubMed
-
- Kraytsberg Y., Kudryavtseva E., McKee A.C., Geula C., Kowall N.W., Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 2006;38:518–520. - PubMed
-
- Bender A., Krishnan K.J., Morris C.M., Taylor G.A., Reeve A.K., Perry R.H., Jaros E., Hersheson J.S., Betts J., Klopstock T., et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006;38:515–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

