Interaction of egocentric and world-centered reference frames in the rat posterior parietal cortex
- PMID: 24741034
- PMCID: PMC3988403
- DOI: 10.1523/JNEUROSCI.0511-14.2014
Interaction of egocentric and world-centered reference frames in the rat posterior parietal cortex
Abstract
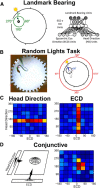
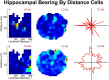
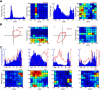
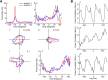
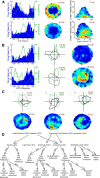
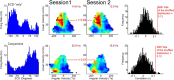
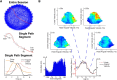
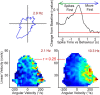
Navigation requires coordination of egocentric and allocentric spatial reference frames and may involve vectorial computations relative to landmarks. Creation of a representation of target heading relative to landmarks could be accomplished from neurons that encode the conjunction of egocentric landmark bearings with allocentric head direction. Landmark vector representations could then be created by combining these cells with distance encoding cells. Landmark vector cells have been identified in rodent hippocampus. Given remembered vectors at goal locations, it would be possible to use such cells to compute trajectories to hidden goals. To look for the first stage in this process, we assessed parietal cortical neural activity as a function of egocentric cue light location and allocentric head direction in rats running a random sequence to light locations around a circular platform. We identified cells that exhibit the predicted egocentric-by-allocentric conjunctive characteristics and anticipate orienting toward the goal.
Keywords: allocentric; head direction; landmark navigation; posterior parietal cortex; spatial navigation; spatial orientation.
Figures




References
-
- Adams AD, Forrester JM. The projection on the rats visual field on the cerebral cortex. Q J Exp Physiol. 1968;53:327–336. - PubMed
-
- Berens P. CircStat: a MATLAB toolbox for circular statistics. J Stat Softw. 2009;31:1–21.
