Rapid developmental emergence of stable depolarization during wakefulness by inhibitory balancing of cortical network excitability
- PMID: 24741038
- PMCID: PMC3988407
- DOI: 10.1523/JNEUROSCI.3659-13.2014
Rapid developmental emergence of stable depolarization during wakefulness by inhibitory balancing of cortical network excitability
Abstract
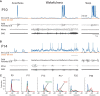
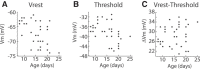
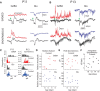
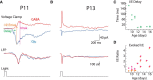
The ability to generate behaviorally appropriate cortical network states is central to sensory perception and plasticity, but little is known about the timing and mechanisms of their development. I paired intracellular and extracellular recordings in the visual cortex of awake infant rats to determine the synaptic and circuit mechanisms regulating the development of a key network state, the persistent and stable subthreshold membrane potential (Vm) depolarization associated with wakefulness/alertness in cortical networks, called the "desynchronized" or "activated" state. Current-clamp recordings reveal that the desynchronized state is absent during the first 2 postnatal weeks, despite behavioral wakefulness. During this period, Vm remains at the resting membrane potential >80% of the time, regardless of behavioral state. Vm dynamics during spontaneous or light-evoked activity were highly variable, contained long-duration supratheshold plateau potentials, and high spike probability, suggesting an unstable and hyperexcitable early cortical network. Voltage-clamp recordings reveal that effective feedforward inhibition is absent at these early ages despite the presence of feedback inhibition. Stable membrane depolarization during wakefulness finally emerges 1-2 d before eye opening and is statistically indistinguishable from that in adults within days. Reduced cortical excitability, fast feedforward inhibition, and the slow cortical oscillation appear simultaneously with stable depolarization, suggesting that an absence of inhibitory balance during early development prevents the expression of the active state and hence a normal wakeful state in early cortex. These observations identify feedforward inhibition as a potential key regulator of cortical network activity development.
Figures


References
-
- André M, Lamblin MD, d'Allest AM, Curzi-Dascalova L, Moussalli-Salefranque F, S Nguyen The T, Vecchierini-Blineau MF, Wallois F, Walls-Esquivel E, Plouin P. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiol Clin. 2010;40:59–124. doi: 10.1016/j.neucli.2010.02.002. - DOI - PubMed
-
- Barnard KE. Beginning rhythms: the emerging process of sleep wake behaviors and self-regulation. Seattle, WA: NCAST, University of Washington; 1999.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
