Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus
- PMID: 24741039
- PMCID: PMC4298648
- DOI: 10.1523/JNEUROSCI.4861-12.2014
Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus
Abstract
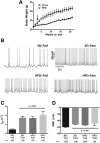
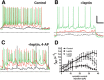
The hypothalamic arcuate nucleus (ARH) is a brain region critical for regulation of food intake and a primary area for the action of leptin in the CNS. In lean mice, the adipokine leptin inhibits neuropeptide Y (NPY) and agouti-related peptide (AgRP) neuronal activity, resulting in decreased food intake. Here we show that diet-induced obesity in mice is associated with persistent activation of NPY neurons and a failure of leptin to reduce the firing rate or hyperpolarize the resting membrane potential. However, the molecular mechanism whereby diet uncouples leptin's effect on neuronal excitability remains to be fully elucidated. In NPY neurons from lean mice, the Kv channel blocker 4-aminopyridine inhibited leptin-induced changes in input resistance and spike rate. Consistent with this, we found that ARH NPY neurons have a large, leptin-sensitive delayed rectifier K(+) current and that leptin sensitivity of this current is blunted in neurons from diet-induced obese mice. This current is primarily carried by Kv2-containing channels, as the Kv2 channel inhibitor stromatoxin-1 significantly increased the spontaneous firing rate in NPY neurons from lean mice. In HEK cells, leptin induced a significant hyperpolarizing shift in the voltage dependence of Kv2.1 but had no effect on the function of the closely related channel Kv2.2 when these channels were coexpressed with the long isoform of the leptin receptor LepRb. Our results suggest that dynamic modulation of somatic Kv2.1 channels regulates the intrinsic excitability of NPY neurons to modulate the spontaneous activity and the integration of synaptic input onto these neurons in the ARH.
Keywords: Kv channels; Kv2.1; arcuate nucleus; hypothalamus; leptin; neuropeptide Y.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
