Mechanisms of coordination in distributed neural circuits: encoding coordinating information
- PMID: 24741053
- PMCID: PMC6608233
- DOI: 10.1523/JNEUROSCI.2670-13.2014
Mechanisms of coordination in distributed neural circuits: encoding coordinating information
Abstract
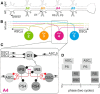
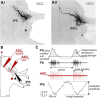
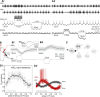
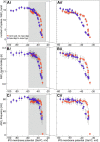
We describe synaptic connections through which information essential for encoding efference copies reaches two coordinating neurons in each of the microcircuits that controls limbs on abdominal segments of the crayfish, Pacifastacus leniusculus. In each microcircuit, these coordinating neurons fire bursts of spikes simultaneously with motor neurons. These bursts encode timing, duration, and strength of each motor burst. Using paired microelectrode recordings, we demonstrate that one class of nonspiking neurons in each microcircuit's pattern-generating kernel--IPS--directly inhibits the ASCE coordinating neuron that copies each burst in power-stroke (PS) motor neurons. This inhibitory synapse parallels IPS's inhibition of the same PS motor neurons. Using a disynaptic pathway to control its membrane potential, we demonstrate that a second type of nonspiking interneuron in the pattern-generating kernel--IRSh--inhibits the DSC coordinating neuron that copies each burst in return-stroke (RS) motor neurons. This inhibitory synapse parallels IRS's inhibition of the microcircuit's RS motor neurons. Experimental changes in the membrane potential of one IPS or one IRSh neuron simultaneously changed the strengths of motor bursts, durations, numbers of spikes, and spike frequency in the simultaneous ASCE and DSC bursts. ASCE and DSC coordinating neurons link the segmentally distributed microcircuits into a coordinated system that oscillates with the same period and with stable phase differences. The inhibitory synapses from different pattern-generating neurons that parallel their inhibition of different sets of motor neurons enable ASCE and DSC to encode details of each oscillation that are necessary for stable, adaptive synchronization of the system.
Keywords: central pattern generators; efference copy; locomotion; swimmeret rhythm; synaptic range.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
