Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function in vivo
- PMID: 24741067
- PMCID: PMC4058109
- DOI: 10.1242/jcs.134833
Cadherin-11 regulates both mesenchymal stem cell differentiation into smooth muscle cells and the development of contractile function in vivo
Abstract
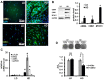
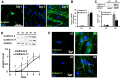
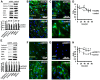
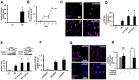
Although soluble factors, such as transforming growth factor β1 (TGF-β1), induce mesenchymal stem cell (MSC) differentiation towards the smooth muscle cell (SMC) lineage, the role of adherens junctions in this process is not well understood. In this study, we found that cadherin-11 but not cadherin-2 was necessary for MSC differentiation into SMCs. Cadherin-11 regulated the expression of TGF-β1 and affected SMC differentiation through a pathway that was dependent on TGF-β receptor II (TGFβRII) but independent of SMAD2 or SMAD3. In addition, cadherin-11 activated the expression of serum response factor (SRF) and SMC proteins through the Rho-associated protein kinase (ROCK) pathway. Engagement of cadherin-11 increased its own expression through SRF, indicative of the presence of an autoregulatory feedback loop that committed MSCs to the SMC fate. Notably, SMC-containing tissues (such as aorta and bladder) from cadherin-11-null (Cdh11(-/-)) mice showed significantly reduced levels of SMC proteins and exhibited diminished contractility compared with controls. This is the first report implicating cadherin-11 in SMC differentiation and contractile function in vitro as well as in vivo.
Keywords: Adherens junctions; Cell–cell adhesion; Differentiation; Mesenchymal stem cells; Smooth muscle cells; Vascular contractility.
© 2014. Published by The Company of Biologists Ltd.
Figures



References
-
- Bohnenpoll T., Bettenhausen E., Weiss A. C., Foik A. B., Trowe M. O., Blank P., Airik R., Kispert A. (2013). Tbx18 expression demarcates multipotent precursor populations in the developing urogenital system but is exclusively required within the ureteric mesenchymal lineage to suppress a renal stromal fate. Dev. Biol. 380, 25–36 10.1016/j.ydbio.2013.04.036 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

