Characterization of the nucleocytoplasmic shuttle of the matrix protein of influenza B virus
- PMID: 24741102
- PMCID: PMC4054458
- DOI: 10.1128/JVI.00794-14
Characterization of the nucleocytoplasmic shuttle of the matrix protein of influenza B virus
Abstract
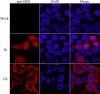
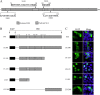
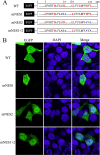
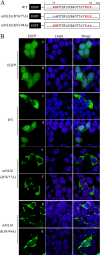
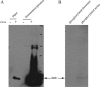
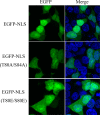
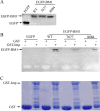
Influenza B virus is an enveloped negative-strand RNA virus that contributes considerably to annual influenza illnesses in human. The matrix protein of influenza B virus (BM1) acts as a cytoplasmic-nuclear shuttling protein during the early and late stages of infection. The mechanism of this intracellular transport of BM1 was revealed through the identification of two leucine-rich CRM1-dependent nuclear export signals (NESs) (3 to 14 amino acids [aa] and 124 to 133 aa), one bipartite nuclear localization signal (NLS) (76 to 94 aa), and two phosphorylation sites (80T and 84S) in BM1. The biological function of the NLS and NES regions were determined through the observation of the intracellular distribution of enhanced green fluorescent protein (EGFP)-tagged signal peptides, and wild-type, NES-mutant, and NLS-mutant EGFP-BM1. Furthermore, the NLS phosphorylation sites 80T and 84S, were found to be required for the nuclear accumulation of EGFP-NLS and for the efficient binding of EGFP-BM1 to human importin-α1. Moreover, all of these regions/sites were required for the generation of viable influenza B virus in a 12-plasmid virus rescue system.
Importance: This study expands our understanding of the life cycle of influenza B virus by defining the dynamic mechanism of the nucleocytoplasmic shuttle of BM1 and could provide a scientific basis for the development of antiviral medication.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Nucleocytoplasmic Shuttling of Porcine Parvovirus NS1 Protein Mediated by the CRM1 Nuclear Export Pathway and the Importin α/β Nuclear Import Pathway.J Virol. 2022 Jan 12;96(1):e0148121. doi: 10.1128/JVI.01481-21. Epub 2021 Oct 13. J Virol. 2022. PMID: 34643426 Free PMC article.
-
Characteristics of nucleocytoplasmic transport of H1N1 influenza A virus nuclear export protein.J Virol. 2014 Jul;88(13):7455-63. doi: 10.1128/JVI.00257-14. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741105 Free PMC article.
-
Apoptin nuclear accumulation is modulated by a CRM1-recognized nuclear export signal that is active in normal but not in tumor cells.Cancer Res. 2005 Aug 15;65(16):7059-64. doi: 10.1158/0008-5472.CAN-05-1370. Cancer Res. 2005. PMID: 16103052
-
The nucleocytoplasmic transport of viral proteins.Virol Sin. 2010 Apr;25(2):79-85. doi: 10.1007/s12250-010-3099-z. Epub 2010 Apr 9. Virol Sin. 2010. PMID: 20960304 Free PMC article. Review.
-
AMPK Localization: A Key to Differential Energy Regulation.Int J Mol Sci. 2021 Oct 10;22(20):10921. doi: 10.3390/ijms222010921. Int J Mol Sci. 2021. PMID: 34681581 Free PMC article. Review.
Cited by
-
Multiple RNA virus matrix proteins interact with SLD5 to manipulate host cell cycle.J Gen Virol. 2021 Dec;102(12):001697. doi: 10.1099/jgv.0.001697. J Gen Virol. 2021. PMID: 34882534 Free PMC article.
-
Natural History of Influenza B Virus-Current Knowledge on Treatment, Resistance and Therapeutic Options.Curr Issues Mol Biol. 2023 Dec 26;46(1):183-199. doi: 10.3390/cimb46010014. Curr Issues Mol Biol. 2023. PMID: 38248316 Free PMC article. Review.
-
Genetic characterization and whole-genome sequencing-based genetic analysis of influenza virus in Jining City during 2021-2022.Front Microbiol. 2023 Jun 22;14:1196451. doi: 10.3389/fmicb.2023.1196451. eCollection 2023. Front Microbiol. 2023. PMID: 37426015 Free PMC article.
-
Robust Lys63-Linked Ubiquitination of RIG-I Promotes Cytokine Eruption in Early Influenza B Virus Infection.J Virol. 2016 Jun 24;90(14):6263-6275. doi: 10.1128/JVI.00549-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122586 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

