Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs
- PMID: 24741304
- PMCID: PMC3970945
- DOI: 10.2147/IJN.S53593
Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs
Abstract
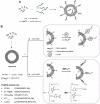
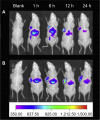
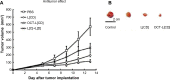
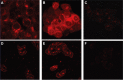
Active targeting by means of drug encapsulated nanoparticles decorated with targeting bioactive moieties represents the next frontier in drug delivery; it reduces drug side effects and increases the therapeutic index. Peptides, based on their chemical and biological properties, could have a prevalent role to direct drug encapsulated nanoparticles, such as liposomes, micelles, or hard nanoparticles, toward the tumor tissues. A considerable number of molecular targets for peptides are either exclusively expressed or overexpressed on both cancer vasculature and cancer cells. They can be classified into three wide categories: integrins; growth factor receptors (GFRs); and G-protein coupled receptors (GPCRs). Therapeutic agents based on nanovectors decorated with peptides targeting membrane receptors belonging to the GPCR family overexpressed by cancer cells are reviewed in this article. The most studied targeting membrane receptors are considered: somatostatin receptors; cholecystokinin receptors; receptors associated with the Bombesin like peptides family; luteinizing hormone-releasing hormone receptors; and neurotensin receptors. Nanovectors of different sizes and shapes (micelles, liposomes, or hard nanoparticles) loaded with doxorubicin or other cytotoxic drugs and externally functionalized with natural or synthetic peptides are able to target the overexpressed receptors and are described based on their formulation and in vitro and in vivo behaviors.
Keywords: active targeting; drug delivery; nanoparticles; receptors binding peptides; supramolecular aggregates.
Figures

References
-
- Richardson PF. Nanotechnology therapeutics in oncology-recent Developments and future outlook. Annu Rep Med Chem. 2012;47:239–252.
-
- Levchenko TS, Hartner WC, Torchilin VP. Liposomes for cardiovascular targeting. Ther Deliv. 2012;3(4):501–514. - PubMed
-
- Maradana MR, Thomas R, O’Sullivan BJ. Targeted delivery of curcumin for treating type 2 diabetes. Mol Nutr Food Res. 2013;57(9):1550–1556. - PubMed
-
- Levesque JP, Winkler IG. It takes nerves to recover from chemotherapy. Nat Med. 2013;19(6):669–671. - PubMed
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

