25th anniversary article: Rational design and applications of hydrogels in regenerative medicine
- PMID: 24741694
- PMCID: PMC3925010
- DOI: 10.1002/adma.201303233
25th anniversary article: Rational design and applications of hydrogels in regenerative medicine
Abstract
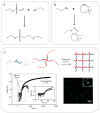
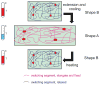
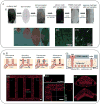
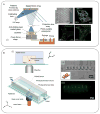
Hydrogels are hydrophilic polymer-based materials with high water content and physical characteristics that resemble the native extracellular matrix. Because of their remarkable properties, hydrogel systems are used for a wide range of biomedical applications, such as three-dimensional (3D) matrices for tissue engineering, drug-delivery vehicles, composite biomaterials, and as injectable fillers in minimally invasive surgeries. In addition, the rational design of hydrogels with controlled physical and biological properties can be used to modulate cellular functionality and tissue morphogenesis. Here, the development of advanced hydrogels with tunable physiochemical properties is highlighted, with particular emphasis on elastomeric, light-sensitive, composite, and shape-memory hydrogels. Emerging technologies developed over the past decade to control hydrogel architecture are also discussed and a number of potential applications and challenges in the utilization of hydrogels in regenerative medicine are reviewed. It is anticipated that the continued development of sophisticated hydrogels will result in clinical applications that will improve patient care and quality of life.
Figures












References
-
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Adv Mater. 2006;18:1345.
-
- Seliktar D. Science. 2012;336:1124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EB012726/EB/NIBIB NIH HHS/United States
- R01 EB012597/EB/NIBIB NIH HHS/United States
- EB00246/EB/NIBIB NIH HHS/United States
- EB012597/EB/NIBIB NIH HHS/United States
- U54 CA143837/CA/NCI NIH HHS/United States
- DE019024/DE/NIDCR NIH HHS/United States
- R01 HL092836/HL/NHLBI NIH HHS/United States
- EB008392/EB/NIBIB NIH HHS/United States
- U54-CA-143837/CA/NCI NIH HHS/United States
- R01 EB000246/EB/NIBIB NIH HHS/United States
- DE021468/DE/NIDCR NIH HHS/United States
- R01 DE021468/DE/NIDCR NIH HHS/United States
- HL099073/HL/NHLBI NIH HHS/United States
- R01 AR057837/AR/NIAMS NIH HHS/United States
- HL092836/HL/NHLBI NIH HHS/United States
- R21 EB012726/EB/NIBIB NIH HHS/United States
- R01 HL099073/HL/NHLBI NIH HHS/United States
- AR057837/AR/NIAMS NIH HHS/United States
- RL1 DE019024/DE/NIDCR NIH HHS/United States
- R01 EB008392/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

