Identification of a strawberry flavor gene candidate using an integrated genetic-genomic-analytical chemistry approach
- PMID: 24742080
- PMCID: PMC4023330
- DOI: 10.1186/1471-2164-15-217
Identification of a strawberry flavor gene candidate using an integrated genetic-genomic-analytical chemistry approach
Abstract
Background: There is interest in improving the flavor of commercial strawberry (Fragaria × ananassa) varieties. Fruit flavor is shaped by combinations of sugars, acids and volatile compounds. Many efforts seek to use genomics-based strategies to identify genes controlling flavor, and then designing durable molecular markers to follow these genes in breeding populations. In this report, fruit from two cultivars, varying for presence-absence of volatile compounds, along with segregating progeny, were analyzed using GC/MS and RNAseq. Expression data were bulked in silico according to presence/absence of a given volatile compound, in this case γ-decalactone, a compound conferring a peach flavor note to fruits.
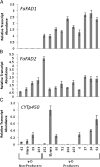
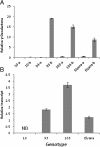
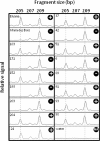
Results: Computationally sorting reads in segregating progeny based on γ-decalactone presence eliminated transcripts not directly relevant to the volatile, revealing transcripts possibly imparting quantitative contributions. One candidate encodes an omega-6 fatty acid desaturase, an enzyme known to participate in lactone production in fungi, noted here as FaFAD1. This candidate was induced by ripening, was detected in certain harvests, and correlated with γ-decalactone presence. The FaFAD1 gene is present in every genotype where γ-decalactone has been detected, and it was invariably missing in non-producers. A functional, PCR-based molecular marker was developed that cosegregates with the phenotype in F1 and BC1 populations, as well as in many other cultivars and wild Fragaria accessions.
Conclusions: Genetic, genomic and analytical chemistry techniques were combined to identify FaFAD1, a gene likely controlling a key flavor volatile in strawberry. The same data may now be re-sorted based on presence/absence of any other volatile to identify other flavor-affecting candidates, leading to rapid generation of gene-specific markers.
Figures
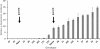


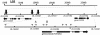

References
-
- Latrasse A. In: Volatile compounds in foods and beverages. Maarse H, editor. New York, NY: Marcel-Decker; 1991. Fruits; pp. 329–387.
-
- Olbricht K, Grafe C, Weiss K, Ulrich D. Inheritance of aroma compounds in a model population of Fragaria × ananassa Duch. Plant Breeding. 2008;15(1):87–93.
-
- Schieberle P, Hofmann T. Evaluation of the character impact odorants in fresh strawberry juice by quantitative measurements and sensory studies on model mixtures. J Agric Food Chem. 1997;15(1):227–232. doi: 10.1021/jf960366o. - DOI
-
- Carrasco B, Hancock J, Beaudry R, Retamales J. Chemical composition and inheritance patterns of aroma in Fragaria x ananassa and Fragaria virginiana progenies. Hort Sci. 2005;15(6):1649–1650.
-
- Bood KG, Zabetakis I. The biosynthesis of strawberry flavor (II): biosynthetic and molecular biology studies. J Food Sci. 2002;15(1):2–8. doi: 10.1111/j.1365-2621.2002.tb11349.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

