Structural and molecular remodeling of dendritic spine substructures during long-term potentiation
- PMID: 24742465
- PMCID: PMC4281348
- DOI: 10.1016/j.neuron.2014.03.021
Structural and molecular remodeling of dendritic spine substructures during long-term potentiation
Abstract
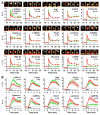
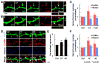
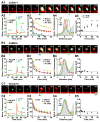
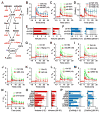
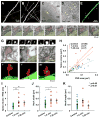
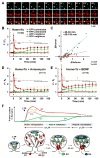
Synapses store information by long-lasting modifications of their structure and molecular composition, but the precise chronology of these changes has not been studied at single-synapse resolution in real time. Here we describe the spatiotemporal reorganization of postsynaptic substructures during long-term potentiation (LTP) at individual dendritic spines. Proteins translocated to the spine in four distinct patterns through three sequential phases. In the initial phase, the actin cytoskeleton was rapidly remodeled while active cofilin was massively transported to the spine. In the stabilization phase, cofilin formed a stable complex with F-actin, was persistently retained at the spine, and consolidated spine expansion. In contrast, the postsynaptic density (PSD) was independently remodeled, as PSD scaffolding proteins did not change their amount and localization until a late protein synthesis-dependent third phase. Our findings show how and when spine substructures are remodeled during LTP and explain why synaptic plasticity rules change over time.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


Comment in
-
How to grow a synapse.Neuron. 2014 Apr 16;82(2):256-7. doi: 10.1016/j.neuron.2014.03.033. Neuron. 2014. PMID: 24742454
References
-
- Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. - PubMed
-
- Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. - PubMed
-
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

