Emergent selectivity for task-relevant stimuli in higher-order auditory cortex
- PMID: 24742467
- PMCID: PMC4048815
- DOI: 10.1016/j.neuron.2014.02.029
Emergent selectivity for task-relevant stimuli in higher-order auditory cortex
Abstract
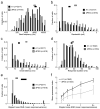
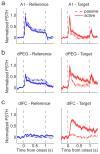
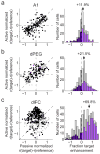
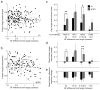
A variety of attention-related effects have been demonstrated in primary auditory cortex (A1). However, an understanding of the functional role of higher auditory cortical areas in guiding attention to acoustic stimuli has been elusive. We recorded from neurons in two tonotopic cortical belt areas in the dorsal posterior ectosylvian gyrus (dPEG) of ferrets trained on a simple auditory discrimination task. Neurons in dPEG showed similar basic auditory tuning properties to A1, but during behavior we observed marked differences between these areas. In the belt areas, changes in neuronal firing rate and response dynamics greatly enhanced responses to target stimuli relative to distractors, allowing for greater attentional selection during active listening. Consistent with existing anatomical evidence, the pattern of sensory tuning and behavioral modulation in auditory belt cortex links the spectrotemporal representation of the whole acoustic scene in A1 to a more abstracted representation of task-relevant stimuli observed in frontal cortex.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cortex. 2005;15:1637–1653. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

