Oridonin inhibits hepatic stellate cell proliferation and fibrogenesis
- PMID: 24742622
- PMCID: PMC4225779
- DOI: 10.1016/j.jss.2014.03.036
Oridonin inhibits hepatic stellate cell proliferation and fibrogenesis
Abstract
Background: Liver fibrosis is a common response to liver injury and, in severe cases, leads to cirrhosis. The hepatic stellate cells (HSCs) become activated after liver injury and play a significant role in fibrogenesis. The activated HSC is characterized by increased proliferation, overexpression of α smooth muscle actin, and excessive production of extracellular matrix (ECM) proteins. Oridonin, a naturally occurring diterpenoid, has been shown to induce apoptosis in liver and gastric cancer cells. However, its effects on the HSC are unknown.
Methods: We tested the effects of oridonin on the activated human and rat HSC lines LX-2 and HSC-T6, and the human hepatocyte cell line C3A. Transforming growth factor β1 (TGF-β1) was used to stimulate LX-2 cells.
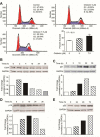
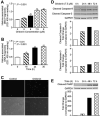
Results: Oridonin significantly inhibited LX-2 and HSC-T6 proliferation. In contrast, oridonin had no antiproliferative effect on C3A cells at our tested range. Oridonin induced apoptosis and S-phase arrest in LX-2 cells. These findings were associated with an increase in p53, p21, p16, and cleaved Poly (ADP-ribose) Polymerase (PARP), and with a decrease in Cyclin-dependent kinase 4 (Cdk4). Oridonin markedly decreased expression of α smooth muscle actin and ECM protein type I collagen and fibronectin, blocked TGF-β1-induced Smad2/3 phosphorylation and type I collagen expression.
Conclusions: Oridonin induces apoptosis and cell cycle arrest involving the p53-p21 pathway in HSC and appears to be nontoxic to hepatocytes. In addition, oridonin suppressed endogenous and TGF-β1-induced ECM proteins. Thus, oridonin may act as a novel agent to prevent hepatic fibrosis.
Keywords: Apoptosis; Liver fibrosis; Oridonin; Stellate cells.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

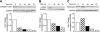

References
-
- Lemoinne S, Cadoret A, El Mourabit H, Thabut D, Housset C. Origins and functions of liver myofibroblasts. Biochim Biophys Acta. 2013;1832:948–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
