Mint proteins are required for synaptic activity-dependent amyloid precursor protein (APP) trafficking and amyloid β generation
- PMID: 24742670
- PMCID: PMC4140894
- DOI: 10.1074/jbc.M113.541003
Mint proteins are required for synaptic activity-dependent amyloid precursor protein (APP) trafficking and amyloid β generation
Abstract
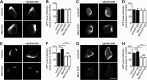
Aberrant amyloid β (Aβ) production plays a causal role in Alzheimer disease pathogenesis. A major cellular pathway for Aβ generation is the activity-dependent endocytosis and proteolytic cleavage of the amyloid precursor protein (APP). However, the molecules controlling activity-dependent APP trafficking in neurons are less defined. Mints are adaptor proteins that directly interact with the endocytic sorting motif of APP and are functionally important in regulating APP endocytosis and Aβ production. We analyzed neuronal cultures from control and Mint knockout neurons that were treated with either glutamate or tetrodotoxin to stimulate an increase or decrease in neuronal activity, respectively. We found that neuronal activation by glutamate increased APP endocytosis, followed by elevated APP insertion into the cell surface, stabilizing APP at the plasma membrane. Conversely, suppression of neuronal activity by tetrodotoxin decreased APP endocytosis and insertion. Interestingly, we found that activity-dependent APP trafficking and Aβ generation were blocked in Mint knockout neurons. We showed that wild-type Mint1 can rescue APP internalization and insertion in Mint knockout neurons. In addition, we found that Mint overexpression increased excitatory synaptic activity and that APP was internalized predominantly to endosomes associated with APP processing. We demonstrated that presenilin 1 (PS1) endocytosis requires interaction with the PDZ domains of Mint1 and that this interaction facilitates activity-dependent colocalization of APP and PS1. These findings demonstrate that Mints are necessary for activity-induced APP and PS1 trafficking and provide insight into the cellular fate of APP in endocytic pathways essential for Aβ production.
Keywords: Adaptor Protein; Alzheimer Disease; Amyloid; Amyloid Precursor Protein (APP); Mint/X11; Synaptic Activity; Trafficking.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures









References
-
- Kamenetz F., Tomita T., Hsieh H., Seabrook G., Borchelt D., Iwatsubo T., Sisodia S., Malinow R. (2003) APP processing and synaptic function. Neuron 37, 925–937 - PubMed
-
- Cirrito J. R., Deane R., Fagan A. M., Spinner M. L., Parsadanian M., Finn M. B., Jiang H., Prior J. L., Sagare A., Bales K. R., Paul S. M., Zlokovic B. V., Piwnica-Worms D., Holtzman D. M. (2005) P-glycoprotein deficiency at the blood-brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J. Clin. Invest. 115, 3285–3290 - PMC - PubMed
-
- Cirrito J. R., Yamada K. A., Finn M. B., Sloviter R. S., Bales K. R., May P. C., Schoepp D. D., Paul S. M., Mennerick S., Holtzman D. M. (2005) Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron 48, 913–922 - PubMed
-
- Haass C., Hung A. Y., Selkoe D. J., Teplow D. B. (1994) Mutations associated with a locus for familial Alzheimer's disease result in alternative processing of amyloid β-protein precursor. J. Biol. Chem. 269, 17741–17748 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

