Impact of inclusion of industry trial results registries as an information source for systematic reviews
- PMID: 24743113
- PMCID: PMC3990559
- DOI: 10.1371/journal.pone.0092067
Impact of inclusion of industry trial results registries as an information source for systematic reviews
Abstract
Background: Clinical trial results registries may contain relevant unpublished information. Our main aim was to investigate the potential impact of the inclusion of reports from industry results registries on systematic reviews (SRs).
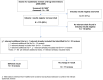
Methods: We identified a sample of 150 eligible SRs in PubMed via backward selection. Eligible SRs investigated randomized controlled trials of drugs and included at least 2 bibliographic databases (original search date: 11/2009). We checked whether results registries of manufacturers and/or industry associations had also been searched. If not, we searched these registries for additional trials not considered in the SRs, as well as for additional data on trials already considered. We reanalysed the primary outcome and harm outcomes reported in the SRs and determined whether results had changed. A "change" was defined as either a new relevant result or a change in the statistical significance of an existing result. We performed a search update in 8/2013 and identified a sample of 20 eligible SRs to determine whether mandatory results registration from 9/2008 onwards in the public trial and results registry ClinicalTrials.gov had led to its inclusion as a standard information source in SRs, and whether the inclusion rate of industry results registries had changed.
Results: 133 of the 150 SRs (89%) in the original analysis did not search industry results registries. For 23 (17%) of these SRs we found 25 additional trials and additional data on 31 trials already included in the SRs. This additional information was found for more than twice as many SRs of drugs approved from 2000 as approved beforehand. The inclusion of the additional trials and data yielded changes in existing results or the addition of new results for 6 of the 23 SRs. Of the 20 SRs retrieved in the search update, 8 considered ClinicalTrials.gov or a meta-registry linking to ClinicalTrials.gov, and 1 considered an industry results registry.
Conclusion: The inclusion of industry and public results registries as an information source in SRs is still insufficient and may result in publication and outcome reporting bias. In addition to an essential search in ClinicalTrials.gov, authors of SRs should consider searching industry results registries.
Conflict of interest statement
Figures
References
-
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R (2008) Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 358: 252–260. - PubMed
-
- Simes RJ (1986) Publication bias: the case for an international registry of clinical trials. J Clin Oncol 4: 1529–1541. - PubMed
-
- Dickersin K, Rennie D (2003) Registering clinical trials. JAMA 290: 516–523. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical